R&D AND INNOVATION
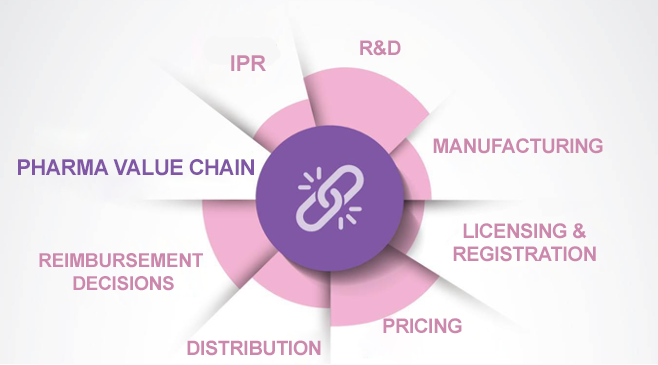
To improve the health level of the society and to increase the global competitiveness of the Turkish pharmaceutical industry, it is evident that the framework regulations on main value chain loops such as R&D, manufacturing, distribution, licensing, registration, manufacturing, pricing, reimbursement, and intellectual property rights, should be handled with a holistic approach and at standards compatible with the targeted developed markets. We can implement the desired leap in the pharmaceutical industry and reach the target of "Türkiye becoming a regional leader in clinical trials" set out in the 11th Development Plan only by establishing an ecosystem that targets the development of the entire value chain.
In this context, AIFD continued to work in 2022 on R&D, clinical research, entrepreneurship ecosystem, sustainability & climate, intellectual property rights and mutual recognition agreements.