CLINICAL TRIALS
Clinical Trials in Türkiye and in the World in numbers
632: Number of clinical trials conducted in 2021 by AIFD members
2 billion 893 million TL (USD 325 million): The amount invested in clinical trials by AIFD members in 2021
Pharmaceutical research and development (R&D) for new drugs and treatments, compared to other sectors, is the most important link in the pharmaceutical value chain due to its long duration, high cost and high risk in terms of return on investment. It is known that R&D investments, to which pharmaceutical companies allocate annually approximately one-fifth of their worldwide prescription drug sales revenues, amount globally to USD 212 billion and that 60 % of this investment is allocated to clinical research.
When the global trends of clinical research, which constitutes the most critical share of the pharmaceutical R&D process, are analysed, it is seen that the regions with the highest number of active studies are North America, Europe and East Asia (led by China and South Korea). The USA, where more than half of the industry-sponsored clinical trials are conducted, is the clear leader, followed by the UK, Spain, Germany and Canada. It should not be surprising that the countries that stand out in clinical research, the most important component of pharmaceutical R&D, are also the leading countries in global pharmaceutical production and trade.
Map: active clinical trials sponsored by the industry
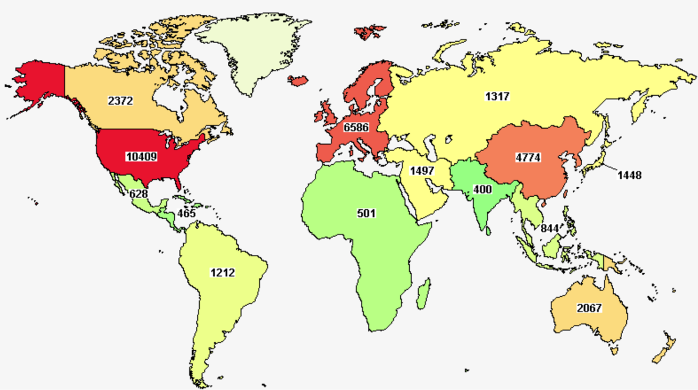
Source: http://www.clinicaltrials.gov; 01 August 2022
When the number of studies in the countries is compared to the size of the population, GDP, and pharmaceutical market according to 2019 figures, it is seen that countries with advanced economies, healthcare systems and clinical research infrastructure such as Belgium, Denmark, the Netherlands, Austria, Singapore, New Zealand and Israel, as well as medium-sized countries, especially Central and Eastern European countries, stand out.
Table 1: Ranking of the first 20 Countries According to the Number of Active Clinical Trials
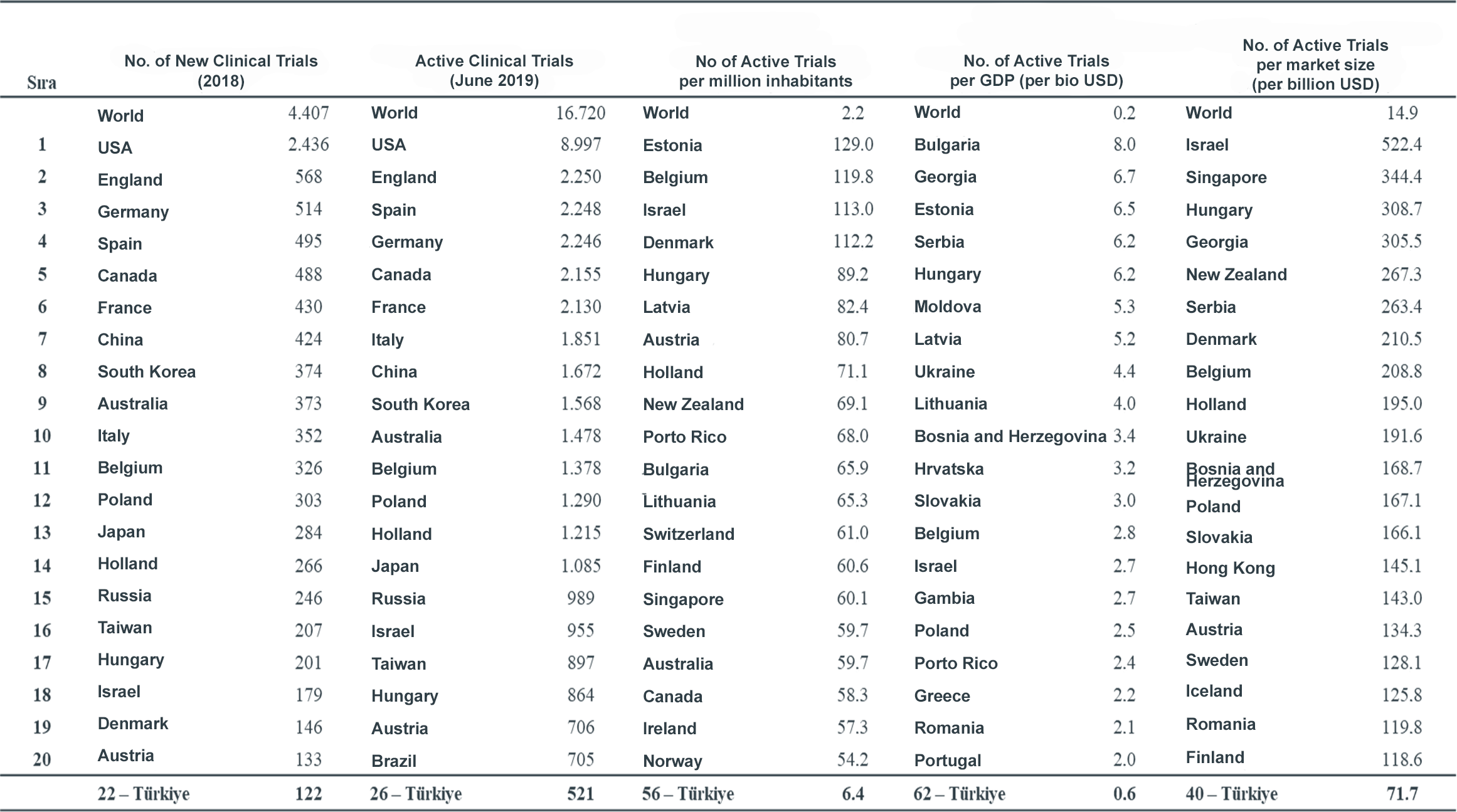
Source: www.clinicaltrials.gov; IQVIA Analysis
IQVIA | The Advantages of Clinical Research Strategy for Turkey | 2020
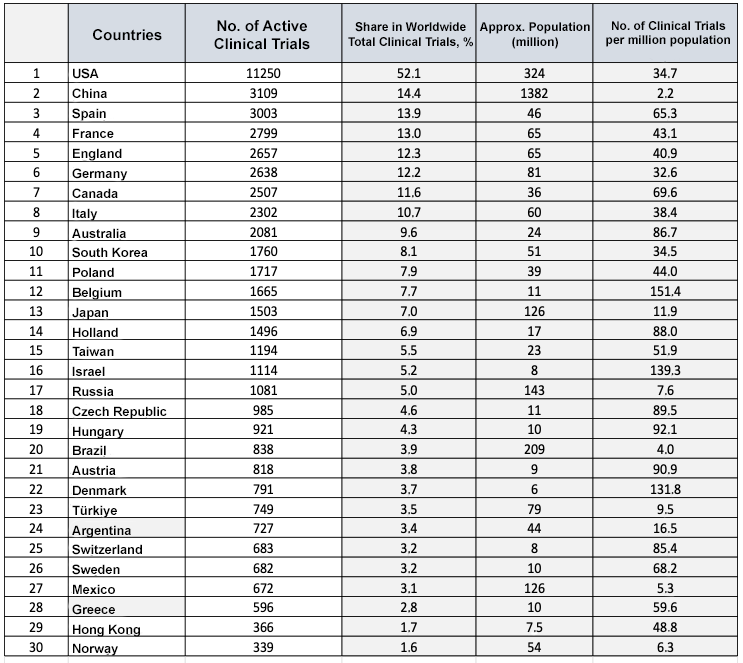
Source: 12 September 2022 www.clinicaltrials.gov
As of 2021, with a market volume of USD 8.1 billion, Türkiye has about six per thousand of the world pharmaceutical market, ranking 19th in the world in terms of pharmaceutical market size and 23rd in terms of industry-sponsored active clinical research. However, when the size of the country is evaluated, the country regresses in the ranking and (according to 2019 figures) ranks 56th in terms of number of clinical trials per population, 62nd in terms of GDP and 40th in terms of the size of the pharmaceutical market. These indicators indicate that Turkish pharma market is not an innovation-driven, high-value pharma market, but rather a more modestly valued manufacturing-based market, and that the country has great clinical research potential. However, with its geographical location, population, disease diversity, healthcare infrastructure, qualified workforce in universities and training and research hospitals, and legislation in line with international regulations, it is clear that Türkiye has significant growth potential to increase its share of global clinical research investment.
In countries similar to Türkiye, where industry-sponsored clinical research is largely conducted by multinational pharmaceutical companies, clinical research should be considered as an important foreign direct investment in terms of the financial value, employment and economic mobility it generates, as well as the positive gains it creates for health, science and patients.
According to the results of the survey carried out among AIFD member companies, which conduct almost all of the industry-sponsored clinical trials in Türkiye, the number of clinical trials of AIFD member companies increased from 2020 to 2021 by 19%, from 529 in 2020 to 632 in 2021. The total investment increased respectively from TL 1 billion 115 million (USD 159 million) to TL 2 billion 893 million (USD 325 million).
Clinical Trials Investment of AIFD Member Companies
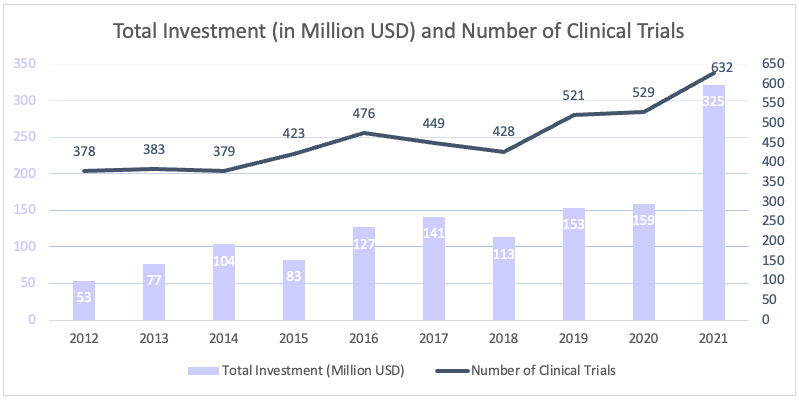
Source: AIFD
The importance of clinical research and the need to realize Türkiye's potential in this field have been emphasized in all public policy documents. In the 11th Development Plan, clinical research, which was already mentioned in the 10th Development Plan, is included in much more detail, and the goal of "making Türkiye a regional leader in clinical research" is set as a target for the public and private sectors.
To help realize this common goal, AIFD, together with TITCK and TÜSEB, led the preparation of a comprehensive and in-depth clinical research report as a roadmap. The report, titled "Benefits of a Clinical Research Strategy for Türkiye", prepared after a broad participatory process with contributions from the public, private sector, and academia, was shared with the public at the TÜSEB Biotechnology Symposium on September 17, 2020. Following the launch of the report, a Core Action Group was established in December 2020, under the coordination of AIFD, consisting of representatives from TITCK, TÜSEB and Clinical Research Association (KAD) and esteemed academics, to implement the 12 key policy recommendations of the report. The report examined the potential benefits of a clinical research strategy for Türkiye, emphasized that such a strategy would enable the state to achieve its goals of economic development, promoting innovation and R&D, creating more added value, reducing the foreign trade deficit and, most importantly, supporting a healthier and disease-resilient society, and made 12 key policy recommendations on how to successfully implement such a strategy, as mentioned above. In order to put these 12 key policy recommendations into practice, AIFD is meeting regularly since December 2020 with valuable stakeholders in the Clinical Trials Action Plan Core Group.
In 2022, AIFD Clinical Research Core Committee organized a series of meetings with the authorities of Clinical Research Centers in our country, AIFD member companies that carry out almost all of the industry-sponsored clinical research in Türkiye, and our valuable stakeholders such as TITCK, TÜSEB and Clinical Research Association (KAD). In addition to the introduction of the invited clinical research center, a series of meetings were organized where the current situation, potential solution suggestions for the problems, and expectations and evaluations regarding the development of cooperation were shared with the sector representatives. In this context, three separate meetings were held in 2022, namely in Cerrahpaşa Clinical Research Excellence Application and Research Center, in Ankara City Hospital and in Ege University.
It is planned to continue these meetings in 2023, and to hold an evaluation meeting in the second half of the year, bringing together the host centers.
Additionally, active support was provided for legislative work in close cooperation with TITCK. In 2022, six legislation documents of great importance for clinical research were published. These are 'Guidelines on Phase I Clinical Research Centers', 'Directive on the Procedures and Principles Regarding the Studies to be Conducted with the Request of the Sponsor within the Scope of Revolving Fund Enterprise', 'General Communiqué on the Law on Supporting Research, Development and Design Activities', 'Regulation Amending the Regulation on Clinical Trials of Pharmaceuticals and Biological Products', 'Guidelines on the Evaluation of Clinical Trials in Exceptional Situations' and 'Regulation Amending the Regulation on Clinical Trials of Pharmaceuticals and Biological Products'.





