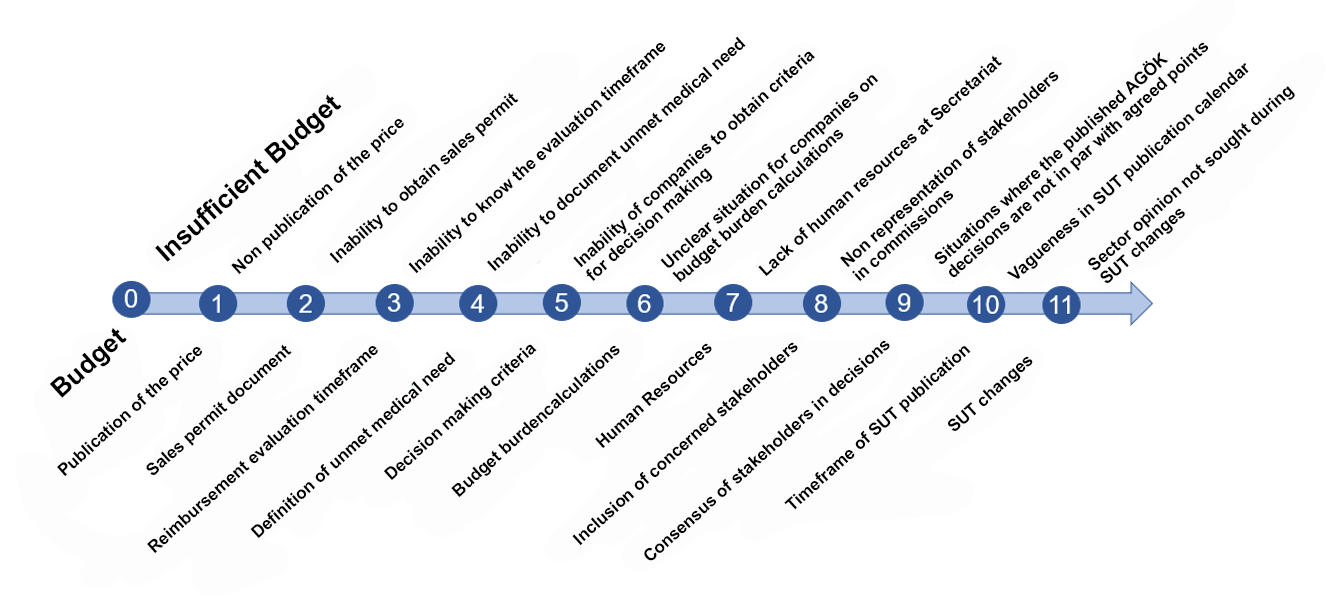
The main problems and proposed solutions regarding reimbursement processes, which were conveyed to the higher authorities of the GSS at the aforementioned meeting, are summarized below:
Assessment 1- The current budget allocated to pharmaceuticals is far from meeting the need, especially for innovative medicines.
Proposed Solution: Evaluate alternative financing potentials for access to innovative medicines,
Assessment 2- There is no foreseeable timetable for the evaluation and finalization of companies' reimbursement applications. This situation also negatively affects the accessibility plans of companies.
Proposed Solution : The timing and criteria of the evaluation processes should be clear and defined in the legislation, and the evaluations should be completed, approved and published in the SUT within 90 days,
Assessment 3- The basic criteria for evaluating the medicines that will be prioritized for the Alternative Reimbursement Commission (AGÖK) process, especially those that will respond to unmet needs, are unclear for companies. There is a need for guidance from the SSI on which medicines are natural candidates for the AGÖK process.
Proposed Solution : The criteria used by the SSI in the evaluation of unmet need should be clear and shareable with companies, the opinion report of the scientific and academic commission should be shared with the company, and the company should be given the right to object,
Assessment 4- The decision-making processes and criteria are not clear and objective for the companies, and in cases of rejection, as the issues determining the decision are not shared with the companies in a reasoned decision, this creates problems for the subsequent application processes of the companies.
Proposed Solution : Multi-stakeholder determination and clarity of objective criteria in decision-making processes,
Assessment 5- The disease data and source for the treatment area, the methodology used are not clearly shared and the basis for the budget burden study are not clear.
Proposed Solution : Between the Commission and the companies, sharing evidence-based data, especially incidence, prevalence and market forecasts, needed to develop alternative reimbursement models
Assessment 6- Difficulties in meeting the current workload of the human resources responsible for the secretariat services of the application and evaluation processes,
Proposed Solution : Increasing existing human resources; capacity building through regular trainings,
Assessment 7- The fact that patients and specialty associations, who are the main rights holders, are not represented in the commissions creates problems in decision-making processes regarding access to medicines.
Proposed Solution : Within the scope of reimbursement processes, increase the involvement of other stakeholders such as scientific and academic commissions, medical specialty associations and patient associations in the commissions in order to make healthier decisions,
Assessment 8- The publication in the SUT of the conditions that the company cannot agree with, or the publication in the SUT of a different version than verbally agreed upon conditions put the companies in a difficult situation.
Proposed Solution : With the legislative amendment to be made, guaranteeing that the conditions agreed in writing between the Institution and the companies will be published in the SUT
Assessment 9- There is no foreseeable timetable for the evaluation and finalization of the reimbursement applications of the companies, and there is no foreseeable timetable for the publication of the decisions in the SUT.
Proposed Solution : Completion of evaluations within 90 days, approval and publication in the SU.,
Assessment 10- Unpredictable changes in SUT practices and inability of supply chains to adapt to these changes.
Proposed Solution : Discussion of the SUT change decisions in advance with the sector; if not possible, notifying the sector a reasonable time before publication and providing a transition period.
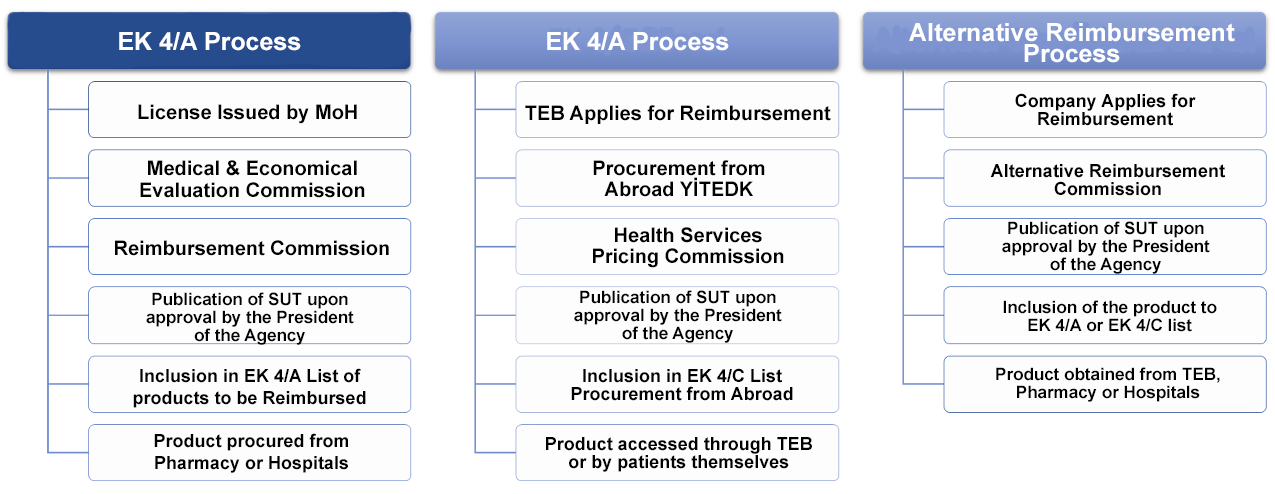
2022 was a period of significant changes in the reimbursement legislation. First of all, the relevant articles of the Social Security Institution General Health Insurance Alternative Reimbursement Regulation published in the Official Gazette dated 10/02/2016 and numbered 29620 (subparagraphs (a) and (d) of the first paragraph of Article 3, subparagraphs (d), (e) and (g) of the first paragraph of Article 6, and subparagraph (4) of the second paragraph (b)) were amended. Subparagraphs (d), (e) and (g) of the first paragraph of Article 3, subparagraphs (d), (e) and (g) of the first paragraph of Article 6, subparagraph (4) of paragraph (b) of the second paragraph and the Confidentiality and Ethical Rules Document in Annex-1), the Council of State (file no. 2021/2950 E.) decided to cancel the relevant articles of the AGÖK Regulation. Taking into account that the administration is obliged to announce the aforementioned decision within 30 days, with the text dated July 6, 2022, the SSI announced the AGÖK Regulation showing the articles annulled by the Council of State in the announcements tab of the institution's website. As of that date, alternative reimbursement became inapplicable due to the annulled articles.
In this context, in line with the guidance of the AIFD Board of Directors, work has been initiated to establish the AIFD position on the AGÖK process, taking into account the need to urgently update the AGÖK legislation, which was designed and implemented to strengthen access to innovative medicines for patients in Turkey, and to make the system operational with the new legislation. In this respect, a unified system proposal has been developed to include the advantages and flexibilities (such as p-volume agreements, price confidentiality) expected from the İGÖK (Pharmaceuticals Reimbursement Commission) system, not independent form İGÖK, and the proposal is planned to be shared with the relevant institution at the next AIFD-GSS workshop in the coming period.
Subsequently, the revised "Social Security Institution Drug Reimbursement Regulation" was published in the Official Gazette dated August 25, 2022 and numbered 31934 (bis), with significant amendments to certain articles. In the revised regulation, AIFD evaluated in detail "the addition of the definition of therapeutic reference group (TR) next to the definition of generic group and the practices regulated in this regard, the powers granted to the Chairman of the TEDK and the Chairman of the Commission regarding prioritization, the prioritization of the first biosimilar if it is below 30% of the price of the reference medicinal product, and unclear statements in the articles regarding the duties of the TEDK" and submitted its requests for amendments to the relevant articles to the SSI in an official letter on October 17, 2022. The amendment also added the provision that discount rates can be hidden, as in alternative reimbursement. On the other hand, it is also known that the revised regulation has been taken to the legal process by other stakeholders within the scope of similar articles.
Reimbursement Schedule:
Reimbursement processes under the responsibility of the Social Security Institution are activities with defined reimbursement periods and timings. A summary table showing the average duration of these periods is given below:
|
2013/I |
2013/II |
2013/III |
|
|
Beginning |
Feb -13 |
May-13 |
Sept -13 |
|
End |
July -14 |
July -14 |
July -14 |
|
Duration in months |
17 |
14 |
10 |
|
2014/I |
2014/II |
2014/III |
|
|
Beginning |
Feb-14 |
May-14 |
Sept-14 |
|
End |
Sept-14 |
Apr-15 |
Jan-16 |
|
Duration in months |
7 |
11 |
16 |
|
2015/I |
2015/II |
2015/III |
|
|
Beginning |
Feb-15 |
May-15 |
Sept-15 |
|
End |
Jun-16 |
Oct-16 |
Oct-16 |
|
Duration in months |
16 |
17 |
13 |
|
2016/I |
2016/II |
2016/III |
|
|
Beginning |
May-16 |
Nov-16 |
Apr-17 |
|
End |
Feb-17 |
Sept-17 |
Jan-18 |
|
Duration in months |
9 |
10 |
9 |
|
2017/I |
2017/II |
|
|
Beginning |
Apr-17 |
Oct-17 |
|
End |
Apr-18 |
May-18 |
|
Duration in months |
12 |
7 |
|
2018/I |
2018/II |
|
|
Beginning |
Mar-18 |
Sept-18 |
|
End |
Dec-18 |
Sept-19 |
|
Duration in months |
9 |
12 |
|
2019/I |
2019/II |
|
|
Beginning |
Jun-19 |
Oct-19 |
|
End |
August-20 |
Dec-20 |
|
Duration in months |
14 |
14 |
|
2020/I |
2020/II |
|
|
Beginning |
May-20 |
Oct-20 |
|
End |
Apr-21 |
Apr-21 |
|
Duration in months |
11 |
6 |
|
2021/I |
2021/II |
|
|
Beginning |
Mar -21 |
Sept -21 |
|
End |
Apr- 22 |
Jun-22 |
|
Duration in months |
13 |
9 |
|
2022/I |
|
|
Beginning |
Apr-22 |
|
End |
August- 22 |
|
Duration in months |
4 |
Legislative Developments on Reimbursement Issues in 2022:
|
21/01/2022 |
The General Directorate of General Health Insurance of the Presidency of the Social Security Institution published an announcement with the title "About the Supply of Medicines and Medical Devices for Patients with Health Reports Due to Chronic Diseases". |
|
8/02/2022 |
"Health Services Pricing Commission Decisions 2022/1 and 2022/2" and "Communiqué on Amendments to the Social Security Institution Health Implementation Communiqué" were published in the Official Gazette dated February 08, 2022 and numbered 31744 (1st bis). |
|
29/03/2022 |
Pursuant to the decision of the Health Services Pricing Commission published in the Official Gazette dated 25.3.2017 and numbered 30018 and the decision of the Extraordinary Medicines Reimbursement Commission dated 08.03.2022; the decisions taken regarding the combined products, which are included in the payment list of the mono forms of each of the active ingredients, was published. Accordingly, medicinal products whose public unit price is not adjusted by the companies in accordance with the aforementioned ratio until the end of the two-month period from the date of the announcement will be published as a list of medicinal products to be inactivated and will enter into force. In the event that the prices of the inactivated medicinal products are adjusted in accordance with the aforementioned rates, they will be activated by the Agency upon the application of the company. At the end of the sixth month from the date of inactivation, it is stated that if no arrangements are made with current prices, they will be removed from the payment list. |
|
8/04/2022 |
The Announcement on the Supply of Medicines for Patients with Health Reports Due to Chronic Diseases has been published, and in the relevant announcement, it has been reported that although the report periods of all medicines other than the drug groups included in the SSI's announcements dated 29.11.2021 and 21.01.2022 have been extended until 31.12.2022, as of 01.06.2022, all medicines can be obtained from SSI contracted pharmacies in return for a physician prescription issued in accordance with the provisions of the Health Implementation Communiqué. |
|
21/04/2022 |
"Health Services Pricing Commission 2022/3 Decisions" and "Communiqué Amending the Social Security Institution Health Implementation Communiqué" were published in the Official Gazette dated April 21, 2022 and numbered 31816. |
|
1/06/2022 |
"Health Services Pricing Commission 2022/4 Decisions" and "Communiqué on Amendments to the Social Security Institution Health Implementation Communiqué" were published in the Official Gazette dated June 1, 2022 and numbered 31853. |
|
2/06/2022 |
June 1, 2022 dated and 31853 numbered "Communiqué on Amendments to the Social Security Institution Health Implementation Communiqué" published in the Official Gazette dated June 1, 2022 and numbered 31853 |
|
3/06/2022 |
The Announcement on the Drug Supply of Patients with a Health Report Due to Chronic Disease has been published, and in the relevant announcement, the report periods of all drugs other than the drug groups included in the SSI's announcements dated 29.11.2021 and 21.01.2022 have been extended until 31.12.2022, and the date of availability of all drugs from SSI contracted pharmacies in return for a physician's prescription issued in accordance with the provisions of the Health Implementation Communiqué as of 01.06.2022, as stated in the SSI announcement dated 08.04.2022, has been updated as 01.07.2022. |
|
30/06/2022 |
With the announcement of SSI dated June 30, 2022; it has been reported that the "Procedures and Principles Regarding Social Security Institution Drug Reimbursement Applications" published on the official website of the Institution on February 12, 2016 and still in force has been repealed and the new "Procedures and Principles Regarding Social Security Institution Drug Reimbursement Applications" has entered into force as of June 30, 2022. |
|
6/07/2022 |
The relevant articles of the Social Security Institution General Health Insurance Alternative Reimbursement Regulation published in the Official Gazette dated 10/02/2016 and numbered 29620 (subparagraphs (a) and (d) of the first paragraph of Article 3, subparagraphs (d), (e) and (g) of the first paragraph of Article 6 and subparagraph (4) of the second paragraph (b) and the Confidentiality and Ethical Rules Document in Annex-1). Subparagraphs (d), (e) and (g) of the first paragraph of Article 3, subparagraphs (d), (e) and (g) of the first paragraph of Article 6, subparagraph (4) of paragraph (b) of the second paragraph and the Confidentiality and Ethical Rules Document in Annex-1), the Council of State (file no. 2021/2950 E.) decided to cancel the relevant articles of the AGÖK Regulation. Taking into account that the administration is obliged to announce the aforementioned decision within 30 days, with the text dated July 6, 2022, the SSI announced the AGÖK regulation showing the articles canceled by the Council of State decision on the announcements tab of the institution's website. |
|
8/07/2022 |
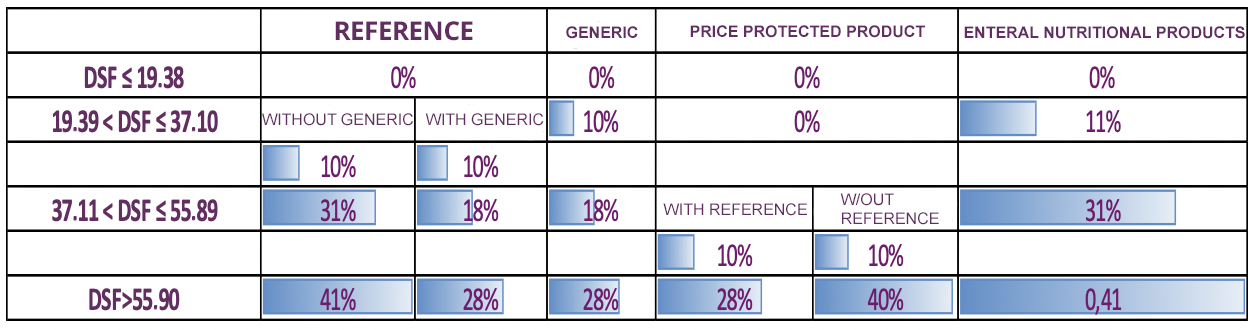
On 8 July 2022, based on the regulation on updating the Euro value used in the Pricing of Medicinal Products for Human Use,published in the Official Gazette dated 24.03.2013 and numbered 28597, it was announced that until the amendment is made in the Social Security Institution Health Implementation Communiqué (SUT), for the warehouse sale prices (DSF) to be applied as of July 9, 2022 the discount rates were announced, determining the discount rates in the article "4.4.1- Discount rates to be applied" |
|
25/08/2022 |
On August 25, 2022, the revised "Social Security Institution Drug Reimbursement Regulation" was published in the Official Gazette dated August 25, 2022 and numbered 31934 (bis). |
|
25/08/2022 |
The "Health Services Pricing Commission Decisions 2022/6 and 2022/7" and the "Communiqué Amending the Social Security Institution Health Implementation Communiqué" were published in the Official Gazette dated August 25, 2022 and numbered 31934 (bis). The main changes in the revised legislation were the search for a MEDULA record of the use of this drug within the scope of the relevant SUT provision for the reimbursement of drugs containing active substances whose condition of use depends on the use of another active substance, and the search for the decision of the relevant commissions for the reimbursement of approved indications for off-label drug use. |
|
14/12/2022 |
In the Official Gazette dated December 14, 2022 and numbered 32043, in line with the Decision on the Amendment of the Decision on the Pricing of Medicinal Products for Human Use (Decision Number: 6546), until the amendment is made in the Social Security Institution Health Implementation Communiqué (SUT), the warehouse sale prices (DSF) to be applied as of December 15, 2022 for the discount rates determined for the discount rates in the article "4.4.1- Discount rates to be applied" of the SUT have been announced. |
|
20/12/2022 |
The Announcement on the Supply of Medicines for Patients with a Health Report Due to Chronic Diseases has been published, and in the relevant announcement, it has been announced that the report periods of all medicines other than the drug groups included in the SSI's announcements dated 29.11.2021 and 21.01.2022 and the medical supplies included in the annex of the announcement dated 20.03.2020 have been extended until 30.06.2023. |
Important meetings, visits and correspondence regarding Reimbursement conducted by AIFD in 2022:
|
20/01/2022 |
In the meeting held with Dr. İrfan Tuncay Alkan, Head of GSS Pharmaceuticals Department, mutual views were exchanged on the future of the pharmaceutical sector with the impact of the developments in our country's economy. |
|
4/03/2022 |
AIFD participated in the 26th TÜSAP Meeting organized with the theme of "Future Vision in Reimbursement Methods in Healthcare".
|
|
5/04/2022 |
During the meeting held with Prof. Dr. Hasan Hüseyin Yıldırım, President of TÜSEB Turkish Health Policy Institute, information was received on the "Implementation of Cost-Effectiveness Analysis for Pharmaceuticals and Determination of National Cost-Effectiveness Threshold" project planned to be carried out in partnership with the General Directorate of SSI GSS and TÜSPE. |
|
5/04/2022 |
A meeting was held with Dr. İrfan Tuncay Alkan, Head of SSI GSS Pharmaceuticals Department, and the issues on the agenda, including the AGÖK processes and the extension of report periods, were discussed during the meeting. |
|
6/04/2022 |
The 2021 results of the WAIT Survey, which reveals the rate of access to innovative products in Turkey in comparison with European countries, have been announced. |
|
10/06/2022 |
At the AIFD-GSS Workshop II, which was held with the participation of AIFD's Board of Directors, the Director General of the GHI and the Head of the Pharmaceuticals Department, AIFD's evaluations and solution proposals regarding reimbursement processes, particularly AGÖK, were conveyed.
|
|
15/06/2022 |
During the meeting with Dr. İrfan Tuncay Alkan, Head of GSS Pharmaceuticals Department, AGÖK processes were discussed in the light of the decision of the Council of State regarding the cancellation of some articles of the AGÖK legislation. |
|
8/07/2022 |
During the meetings with EFPIA officials Tina Taube and Mihai Rotaru, the Equity-Based Tiered Pricing (EBTP) system and the HTA Regulation, which are currently being worked on in the EU, were discussed and the relevant experts were asked to organize information sessions for our SMC members on the current developments on the subject. As a priority, it was decided to plan a session on EBTP by Tina Taube at our next SMC meeting. |
|
15/08/2022 |
In the new "Procedures and Principles for Drug Reimbursement Applications of the Social Security Institution", which entered into force with the announcement of the SSI dated June 30, 2022, our objections regarding the important changes made in the commitment letters included in the file content to be submitted in the applications for inclusion of drugs in the lists have been communicated to the said institution with an official letter signed by AIFD, SURDER and TISD. |
|
22/09/2022 |
A meeting was held with Tuncay Alkan, Head of GSS Department, regarding amendments/additions to the commitments within the scope of the Procedures and Principles on Drug Reimbursement Applications. |
|
23/09/2022 |
Our letter containing AIFD's legal assessments regarding the text of the letter of undertaking attached to the Procedures and Principles for Drug Reimbursement Applications was sent to the GSS. |
|
5/10/2022 |
Officials from TOBB, AIFD, TISD and IEIS paid a visit to Prof. Dr. Gökhan Tuna Öztürk, General Director of GSS and discussed the main problems in the sector, expectations of the sector and possible collaborations with the institution. . |
|
17/10/2022 |
AIFD's legal assessments and requests regarding the amendments introduced to certain articles of the "Social Security Institution Drug Reimbursement Regulation" published in the Official Gazette dated August 25, 2022 and numbered 31934 (bis) were submitted to the SSI in an official letter. |
|
5-9/11/2022 |
The Professional Society for Health Economics and Outcomes Research (ISPOR) organized a program titled " Collaborating Across Borders: Building & Using Evidence to Enable Access" was held in Vienna, and AIFD officials and relevant GHI staff participated in the program online. |
|
23/11/2022 Foto? |
An online evaluation meeting was held to exchange views on the topics discussed in the ISPOR 2022 program, such as pricing and reimbursement systems in Europe, EU Unified HTA Regulation, real life data, and the experiences of our member company representatives who physically participated in the program and the outputs of these developments for our country. |
|
24/11/2022 foto |
The TOBB Pharmaceutical Industry Council Meeting was held with the participation of Prof. Dr. Gökhan Tuna Öztürk, General Director General of General Health Insurance of the Social Security Institution and Dr. İrfan Tuncay Alkan, Head of Pharmaceuticals Department of the SSI. Within the scope of the aforementioned meeting, the problems experienced by the sector in the field of reimbursement and the proposed solutions to these problems were presented in detail and mutual evaluations were made. . |
|
2-3/12/2022 |
AIFD participated to the "Health Technology Assessment III Meeting" organized by the Ministry of Health, Department of Research and Development and Health Technology Assessment. |

