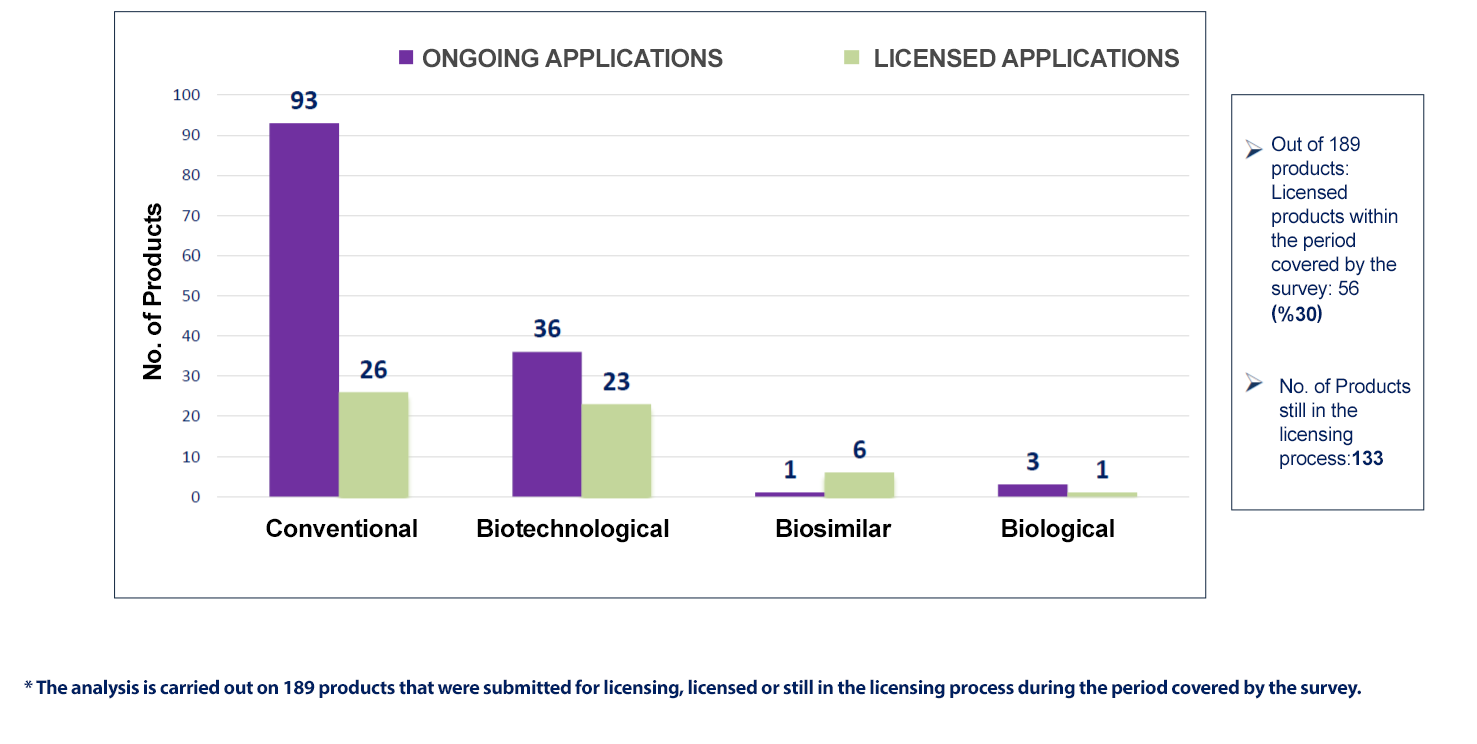
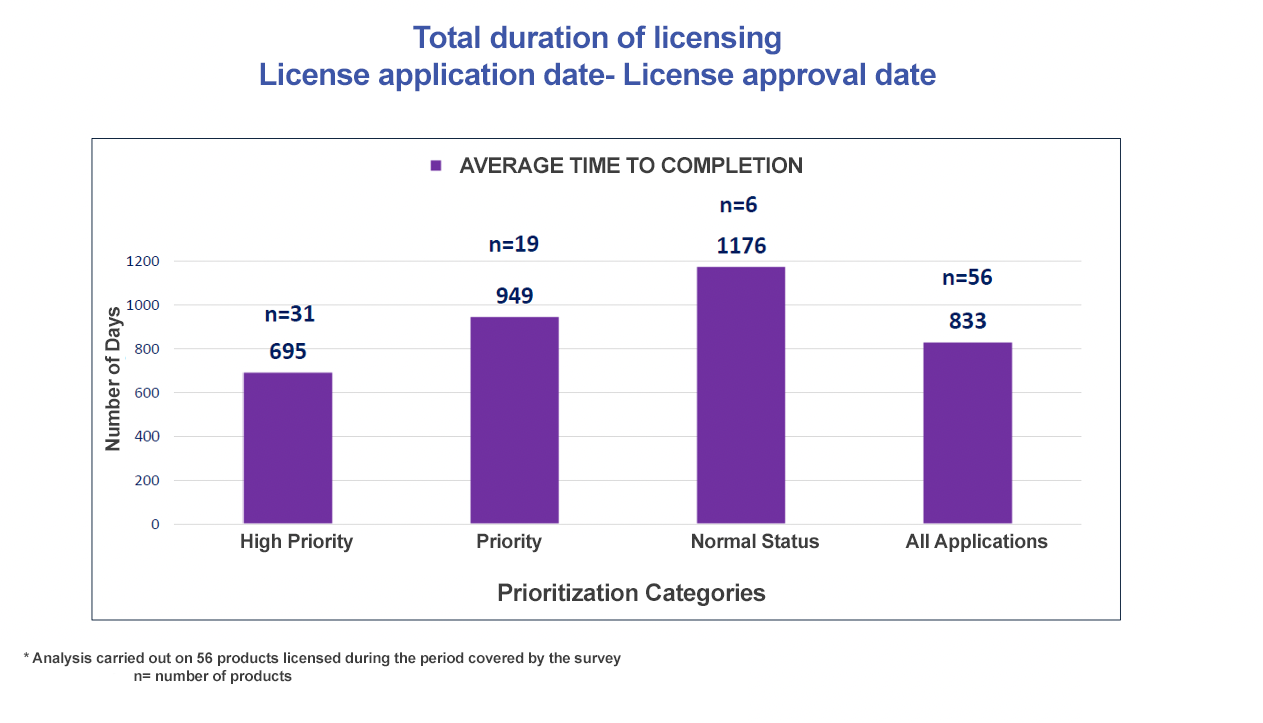
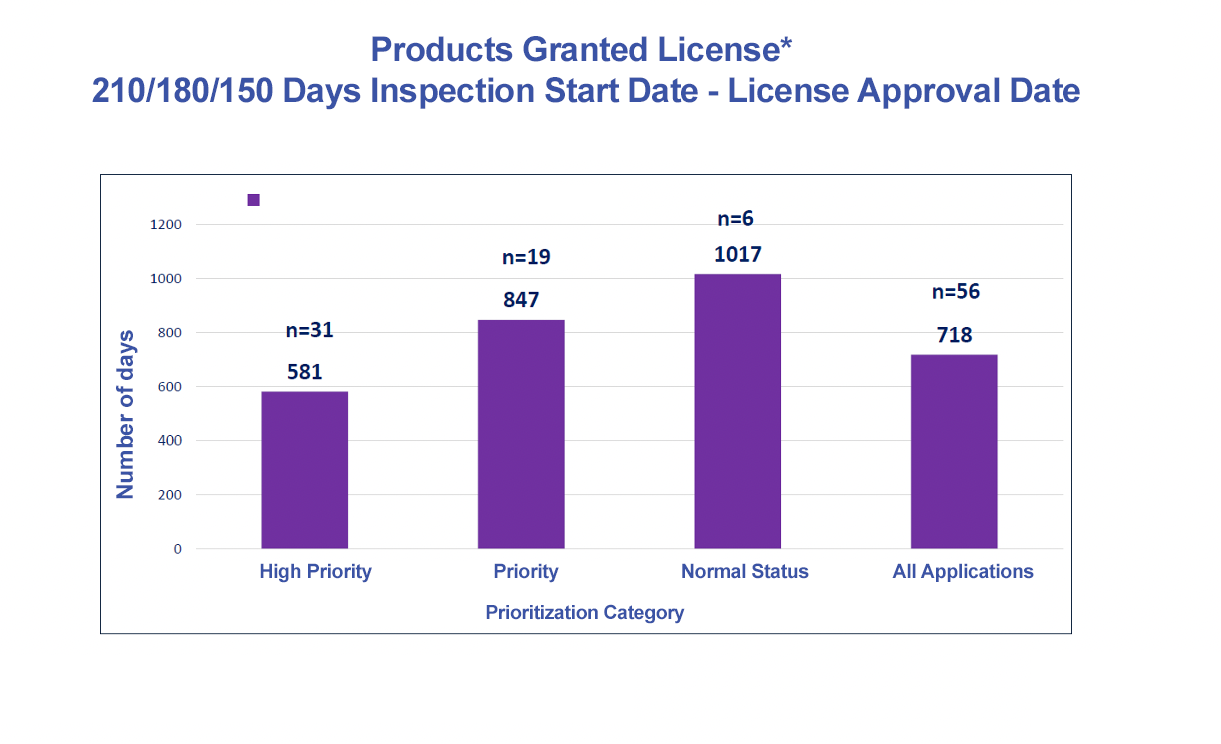
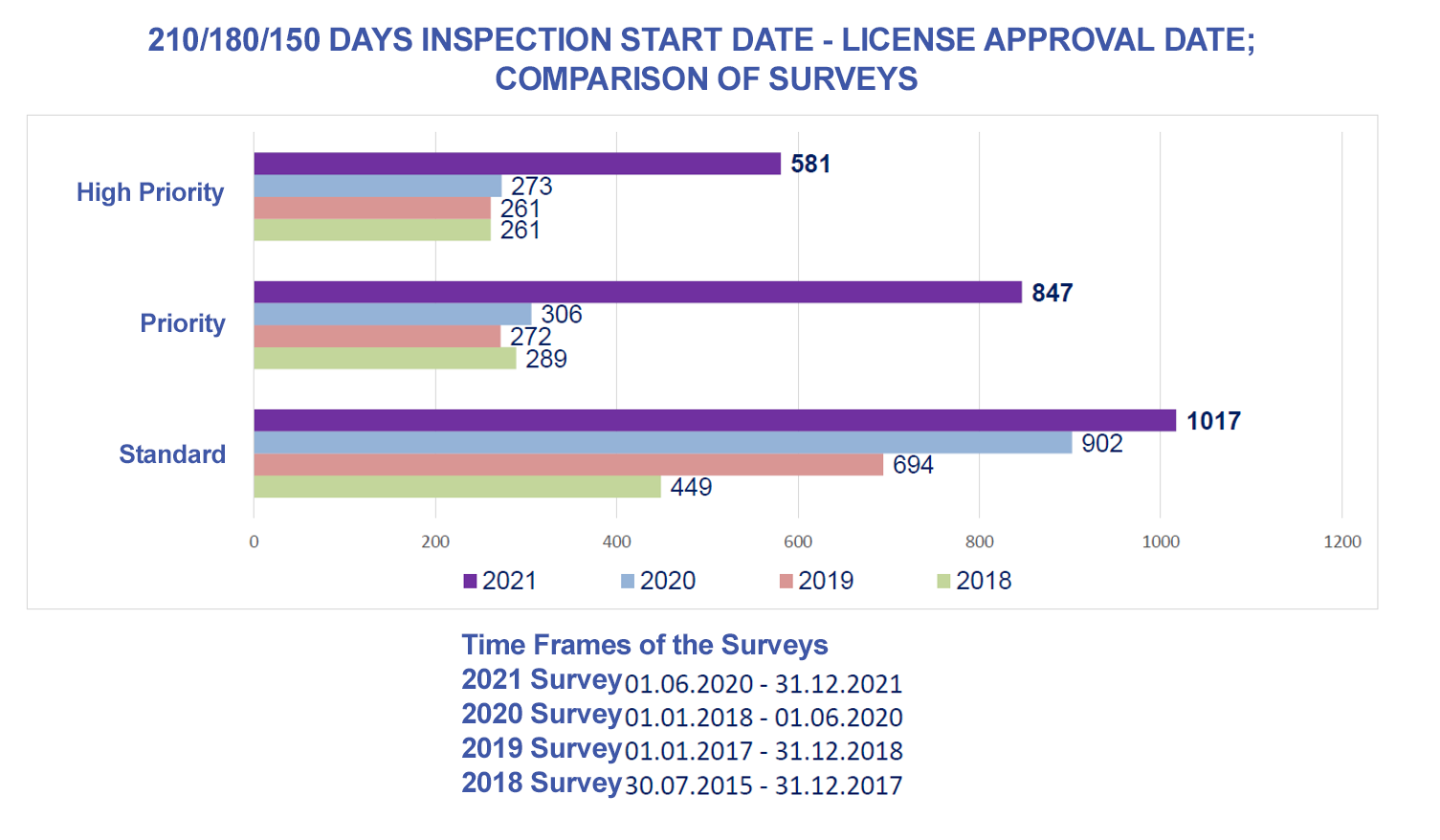
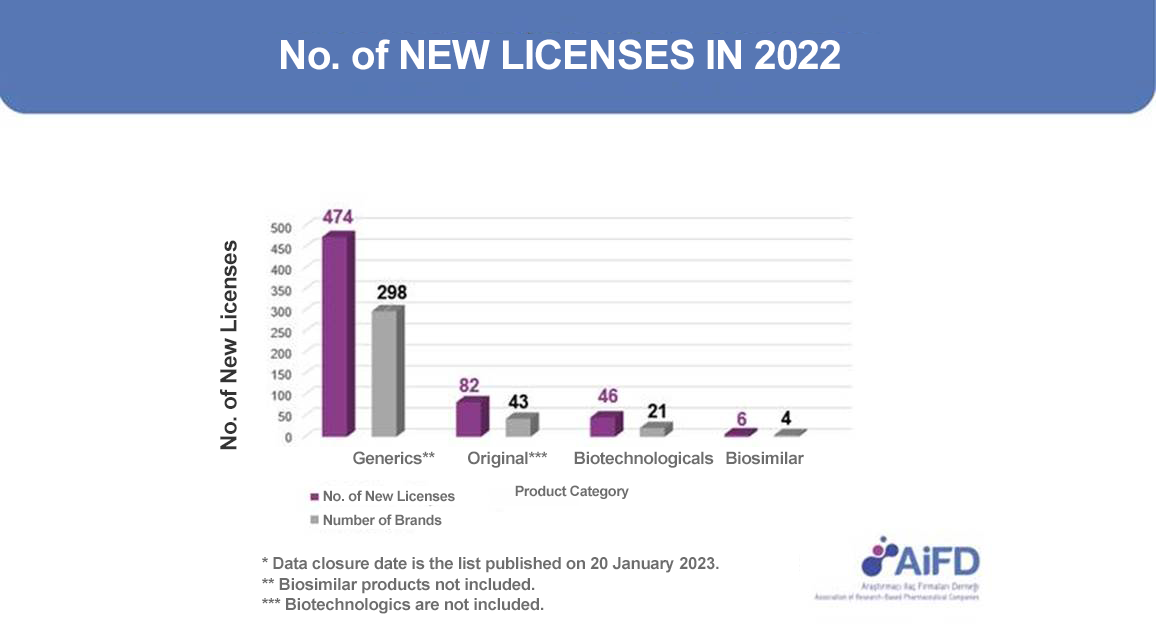
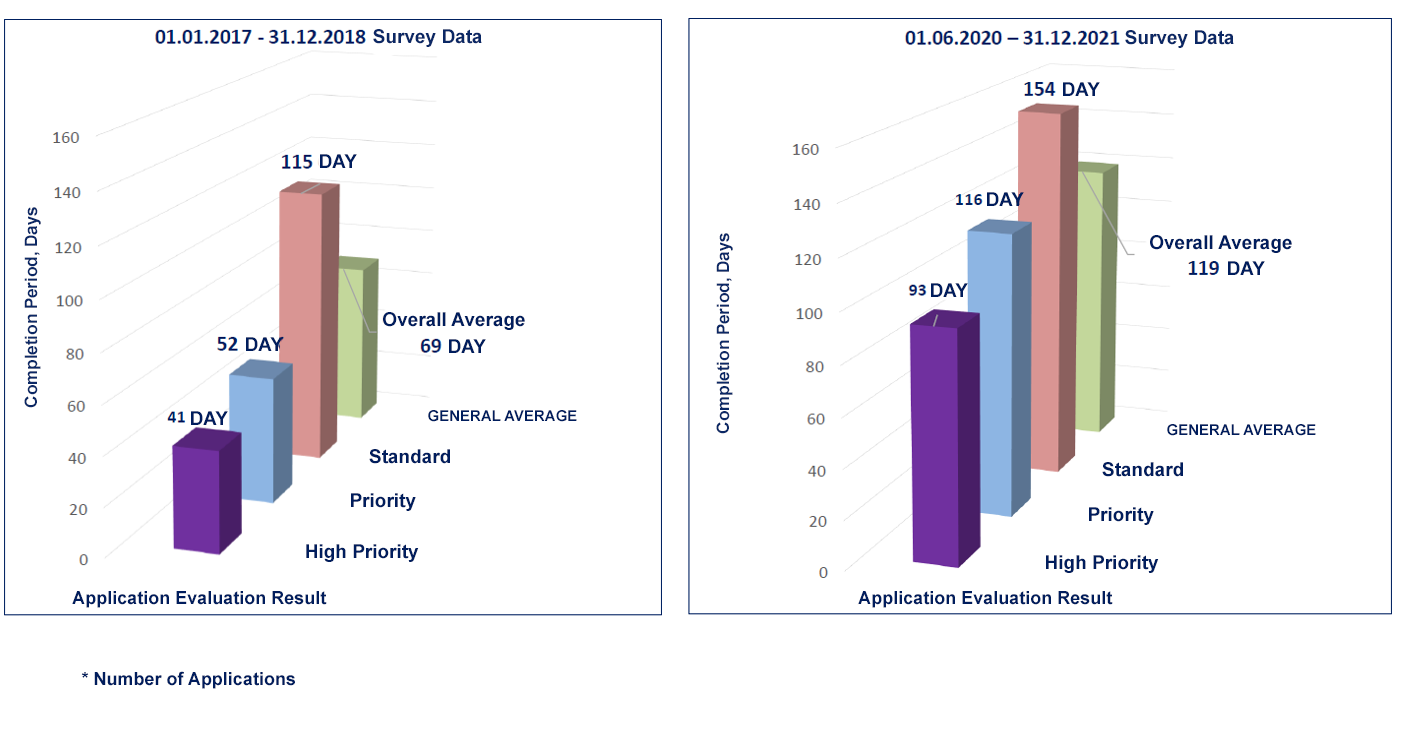
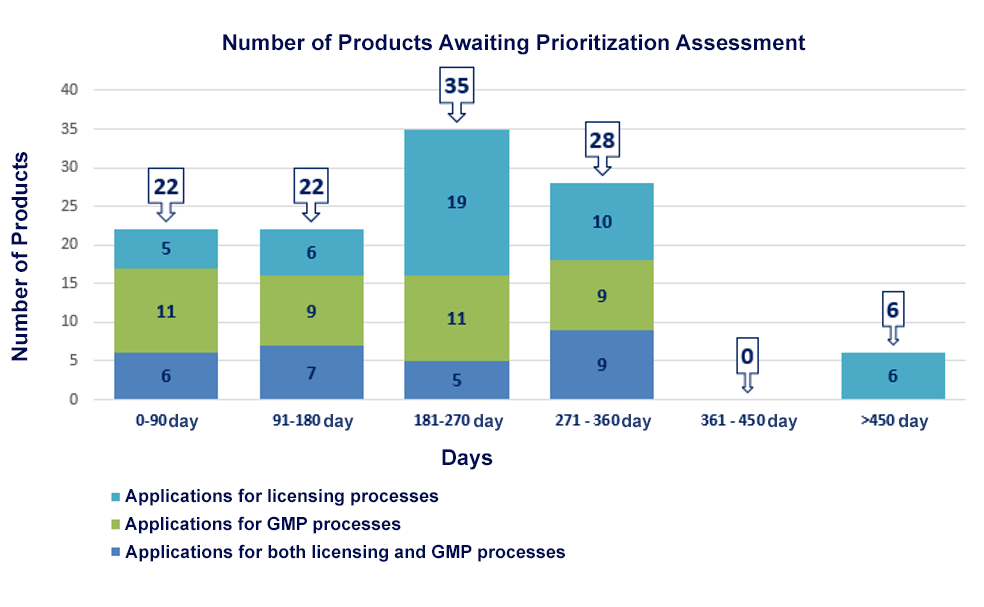
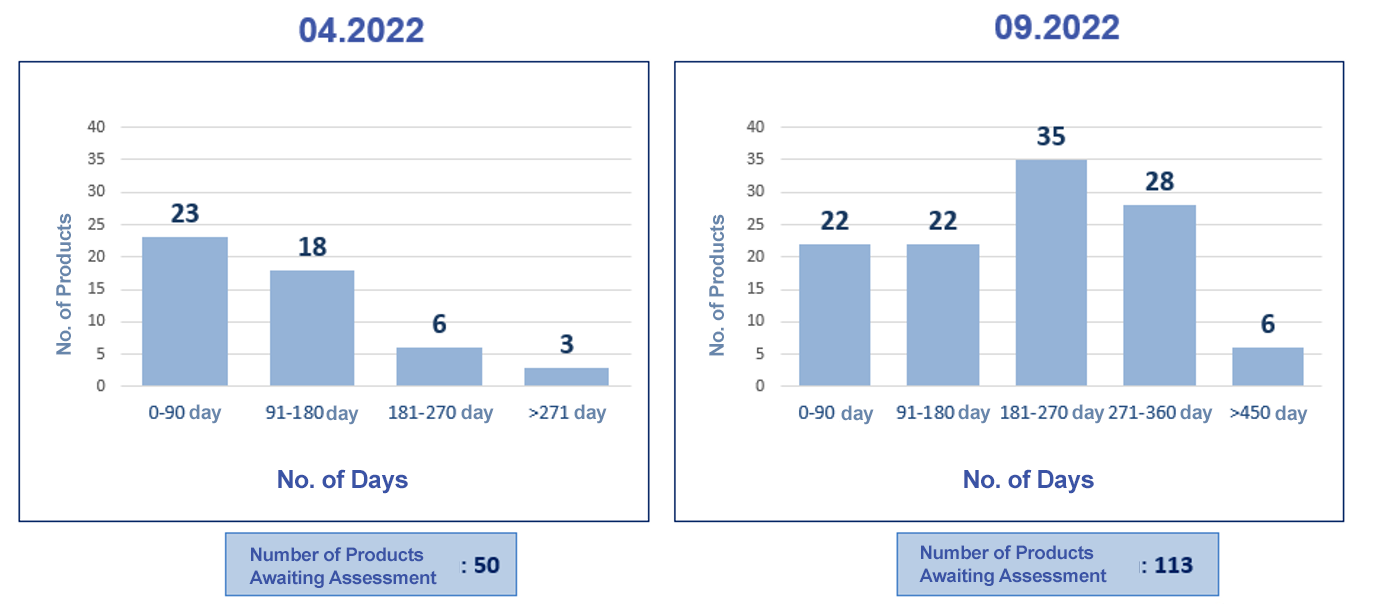
|
PUBLICATION DATE |
ISSUING TITCK DEPARTMENT |
LEGISLATION - PUBLICATION |
|
18/01/2022 |
Pharmaceutical Licensing Dept. |
Application Form and CTD Guide revision within the scope of the Regulation on the Licensing of Medicinal Products for Human Use published on 11.12.2021 + Announcement about Hybrid and Diversification applications |
|
20/01/2022 |
Pharmaceutical Licensing Dept |
*Guidance on Variations in Licensed Medicinal Products for Human Use * Notification/Application Form For Variations in Licensed Medicinal Products for Human Use |
|
26/01/2022 |
Dept. Herbal and Dietary Supplement Products |
Draft Regulation on Permission for Special Medical Nutritional Products |
|
11/02/2022 |
Pharmaceutical Inspection Dept |
Guideline on Serial Release |
|
15/02/2022 |
Pharmaceutical Inspection Dept |
Guideline on the Inspections of Good Distribution Practices |
|
25/02/2022 |
Pharmaceutical Inspection Dept |
* Guidance on Triggers for Audits of Bioavailability/Bioequivalence Studies * Guidance on Triggers for Routine and/or "For a Specific Reason" Inspections |
|
08/03/2022 |
Pharmaceutical Inspection Dept |
Application Guide for Good Clinical Practice Audits |
|
08/03/2022 |
Herbal and Dietary Supplement Products Dept |
Draft Regulation on the Use of Health Declarations |
|
09/03/2022 |
Pharmaceutical Licensing Dept |
Guidance on the Licensing of Allergenic Products |
|
12/03/2022 |
Pharmaceutical Inspection Dept |
Additional Measures to be Implemented During the Pandemic Regarding GMP Audits and Certification Processes Abroad |
|
15/03/2022 |
Herbal and Dietary Supplement Products Dept |
Guidance on License Application for Homeopathic Medicinal Products * Guideline on Packaging and Homeopathic Medicinal Product Information, Readability and Monitoring of Homeopathic Medicinal Products |
|
15/03/2022 |
Strategy Development Department |
Turkish Medicines and Medical Devices Agency Communication Cooperation and Information Sharing Guideline |
|
14/04/2022 |
Pharmaceutical Licensing Dept |
Guidance on the Classification of Diversification Applications and Variation Applications |
|
22/04/2022 |
Pharmaceutical Inspection Dept |
Good Manufacturing Practices (GMP) Guidance for Manufacturers of Medicinal Products for Human Use |
|
22/04/2022 |
Pharmaceutical Inspection Dept |
Guidance on Communication, Organization and Responsibilities of Stakeholders in Market Monitoring Programs |
|
27/04/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Crisis Management Guide in Pharmacovigilance Activities |
|
27/04/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (IFU) Guidelines Module IX Annex I - Training Materials |
|
27/04/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Guide to Good Pharmacovigilance Practices (IFU) Module IX - Risk Minimization Measures: Selection of Tools and Efficacy Indicators |
|
27/04/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (IFU) Guidelines Module VIII - Safety Communication |
|
28/04/2022 |
Pharmaceutical Licensing Dept |
Guideline on Investigation of Bioavailability and Bioequivalence of Medicinal Products for Human Use |
|
11/05/2022 |
Strategy Development Department |
Guideline on Good Regulatory Implementation Practice |
|
07/06/2022 |
Herbal and Dietary Supplement Products Dept |
New Health Declaration Application Draft Guideline |
|
07/06/2022 |
Herbal and Dietary Supplement Products Dept |
Draft Guideline on Packaging Information and Readability of Foods for Special Medical Purposes |
|
10/06/2022 |
Pharmaceutical Inspection Dept |
Regulation Amending the Regulation on Manufacturers of Medicinal Products for Human Use |
|
16/06/2022 |
Pharmaceutical Inspection Dept |
Announcement on "GMP Processes Required for the Authorization of Allergenic Products" |
|
20/06/2022 |
Herbal and Dietary Supplement Products Dept |
* Draft Guidance on the Use of Health Claims * Annex to the Draft Guideline on the Use of Health Declarations |
|
20/06/2022 |
Dept. Herbal and Dietary Supplement Products |
Announcement on “Traditional Herbal Medical Medical Products containing "Hedara Helix L.” |
|
20/06/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (IFU) Guideline Module X - Pre-marketing Benefit/Risk Assessment |
|
20/06/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (IFU) Guideline Module XI - Post-marketing Benefit/Risk Assessment |
|
07/07/2022 |
Dept. Of Analysis and Control Laboratories |
Revision of Sample Acceptance Criteria Procedure |
|
03/08/2022 |
Pharmaceutical Licensing Dept |
Announcement regarding the "Regulation in the Affairs and Procedures of the Department of Drug Licensing due to the COVID-19 Pandemic" (Accordingly, the announcement dated August 26 is no longer valid) |
|
03/08/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Guide to Good Pharmacovigilance Practices Annex I - Definitions (1st revised edition) |
|
09/08/2022 |
Pharmaceutical Inspection Dept |
Guidance on Risk-Based Good Clinical Practice Audits |
|
09/08/2022 |
Pharmaceutical Inspection Dept |
Reliance Guideline for Good Manufacturing Practice Assessments |
|
11/08/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (IFU) Guidelines Module VI - Risk Management Systems (Corrected 1st edition) |
|
11/08/2022 |
Dept. Of Analysis and Control Laboratories |
Guidance on Serial Release of Vaccines and Immune Serums |
|
12/08/2022 |
Pharmaceutical Inspection Dept |
Guidance on Good Distribution Practices (GDP) for Medicinal Products for Human Use |
|
25/08/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (IFU) Guideline Module III -Periodic Benefit/Risk Assessment Report |
|
25/08/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Pharmacovigilance Indicators Guide |
|
26/08/2022 |
Pharmaceutical Licensing Dept |
Announcement on "Prioritization request applications for supply issues of variation applications for licensed Medicinal Products for Human Use" |
|
29/08/2022 |
Dept. Of Rational Drug Use |
Guidance on Donations of Human Medicinal Products from Abroad that are not licensed in Türkiye |
|
02/09/2022 |
Dept of Clinical Research |
Guidance on Reliance Practices in Clinical Trial Applications |
|
02/09/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Draft Guideline on Reliance Practices in Pharmacovigilance Activities (The final Guideline was published on 09.09.2022) |
|
02/09/2022 |
Pharmaceutical Licensing Dept |
Information on Nitrosamines |
|
05/09/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (IFU) Guide Module VII - Signal Management (Revised 1st edition) |
|
07/09/2022 |
Strategy Development Department |
Guidance on the Management of Crises and Emergencies Where Routine Regulatory Processes for Medicinal Products for Human Use cannot be Followed |
|
09/09/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Guidance on Reliance Practices in Pharmacovigilance Activities |
|
12/09/2022 |
Pharmaceutical Inspection Dept |
Internal Communication Guideline of the Medicines Inspection Department (revised guideline published on 24.09.2022) |
|
15/09/2022 |
Pharmaceutical Licensing Dept |
Guidelines on Principles and Processes of Good Evaluation Practices in the Licensing and Post-licensing Procedures of Medicinal Products for Human Use |
|
20/09/2022 |
Pharmaceutical Inspection Dept |
Guidance on Applications for GMP Audits of Overseas Production Facilities |
|
20/09/2022 |
Pharmaceutical Inspection Dept |
Guidance on Centers where Bioavailability and Bioequivalence Studies are conducted |
|
24/09/2022 |
Pharmaceutical Inspection Dept |
Application Guide for Places Assessed under Good Distribution Practices (GDP) |
|
24/09/2022 |
Pharmaceutical Inspection Dept |
Application Guide for Medicinal Product Facilities for Human Use |
|
24/09/2022 |
Pharmaceutical Inspection Dept |
Medicines Inspection Department Internal Communication Guide (Revision.01) |
|
25/09/2022 |
Pharmaceutical Licensing Dept |
Regulation Amending the Regulation on Licensing of Medicinal Products for Human Use |
|
26/09/2022 |
Pharmaceutical Licensing Dept |
Guidance on Reliance Practices Regarding the Authorization Process of Medicinal Products for Human Use |
|
30/09/2022 |
Pharmaceutical Inspection Dept |
Regulation on Serial Release of Vaccines and Immune Serums |
|
12/10/2022 |
Pharmaceutical Licensing Dept |
Announcement on Variation Applications Submitted within the Scope of Reliance for Licensed Medicinal Products for Human Use |
|
13/10/2022 |
Pharmaceutical Inspection Dept |
Regulation on Serial Release of Vaccines and Immune Serums |
|
07/11/2022 |
Pharmaceutical Licensing Dept |
Frequently Asked Questions & Application Considerations Documents have been announced |
|
16/11/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Draft Guideline on Pharmacovigilance System (Final Guideline published on 05.12.2022) |
|
30/11/2022 |
Dept of Analysis ans Control Laboratories |
Regulation on the Duties, Authorities and Responsibilities and Working Procedures and Principles of the National Control Laboratory of the Turkish Medicines and Medical Devices Agency |
|
05/12/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Pharmacovigilance System Guideline (entered into force on 30.11.2022) |
|
05/12/2022 |
Dept. of Pharmacovigilance and Controlled Substances |
Good Pharmacovigilance Practices (GPP) Guideline Module XI - Postmarketing Benefit/Risk Assessment Revision |
|
12/12/2022 |
Pharmaceutical Inspection Dept |
Pharmacovigilance Audit Guide |
|
12/12/2022 |
Pharmaceutical Inspection Dept |
* Revision of Guideline on Good Distribution Practices Audits * Revision of Guideline on Good Distribution Practices for Medicinal Products for Human Use (GDP) * Revision of Application Guideline for Places Assessed under Good Distribution Practices (GDP) |
|
13/12/2022 |
Pharmaceutical Inspection Dept |
Guideline for Communication with External Stakeholders of the Vice Presidency of Audit Services |
|
15/12/2022 |
Pharmaceutical Inspection Dept |
Revision of the Guideline on Human Medicinal Product Facilities Risk Assessment Application |
|
15/12/2022 |
Dept. of Economic Assessments and Pharmaceutical Supply Management |
Announcement on the Cancellation of the Decision on the Extension of the Approval Periods for the Use of Off-label/International Medicinal Products |
|
15/12/2022 |
Pharmaceutical Inspection Dept |
Revision of the Guideline on Serial Release Location Application |
|
15/12/2022 |
Pharmaceutical Inspection Dept |
Revision of the Guideline on Production Location Permit Renewal Application |
|
15/12/2022 |
Assessments and Pharmaceutical Supply Management |
Announcement to update the List of Off-label Medicinal Products that can be used without obtaining TITCK's Additional Approval |
|
16/12/2022 |
Pharmaceutical Licensing Dept |
Regulation Amending the Regulation on Licensing of Medicinal Products for Human Use |
|
21/12/2022 |
Pharmaceutical Inspection Dept |
Revision of Guideline for Application for Medicinal Product Facilities for Human Use |
|
23/12/2022 |
Pharmaceutical Licensing Dept |
Announcement on Pharmaceutical Licensing Agency Company Meetings |
|
30/12/2022 |
Pharmaceutical Inspection Dept |
Guidance on Applications for GMP Audits of Production Facilities in other countries |
In addition to the aforementioned legislation published by TITCK, "Rare Diseases Health Strategy Document and Action Plan" was published on 23/11/2022 by the Directorate General of Health Services, Department of Autism Mental Special Needs and Rare Diseases.
Contacts with Stakeholders
In 2022, meetings were held online and face-to-face within the scope of the issues falling within the fields of activity of the Health Science Policies SMC, and the problems and solution proposals identified by AIFD member companies were shared and the process was effectively monitored.
|
DATE |
RELATED INSTITUTION (S)
|
CONTACT PERSON(S) |
SUBJECT |
|
16/02/2022 |
TITCK Dept. of Analysis and Control Laboratories |
Doç. Dr. Mehmet Kürşat Derici |
Areas where cooperation with AIFD could be contemplated were evaluated. |
|
25/02/2022 |
GHI (GSS) Medicines Dept |
Dr. Tuncay Alkan |
Current issues related to Rare Diseases were evaluated. |
|
02/03/2022 |
TITCK Vice Presidency of Pharmaceuticals and Pharmacy |
Dr. Asım Hocaoğlu |
The revision of the Prioritization Guideline and TITCK participation to DIA 2022 were discussed. |
|
03/03/2022 |
TITCK Dept. of Pharmaceutical Licensing |
Uzm. Ecz. Handan Öztunca |
Evaluations were made on the revision of the Prioritization Guideline, problems encountered in the scope of Summary Product Information (SmPC) and Instructions for Use (KT) applications and also on DIA 2022 Organization. |
|
23/03/2022 |
TITCK Dept. of Pharmaceutical Licensing |
Uzm. Ecz. Handan Öztunca |
Evaluations were made on current issues/problems within the scope of licensing processes. |
|
07/04/2022 |
TITCK Vice Presidency of Pharmaceuticals and Pharmacy, TITCK Dept. of Pharmaceutical Licensing |
Dr. Asım Hocaoğlu, Uzm. Ecz. Handan Öztunca |
The presentation prepared by the AIFD Reliance working group on Reliance Practices was presented and mutually evaluated. |
|
14/04/2022 |
Vice Director of Health Services |
Doç. Dr. Mehmet Gündüz |
Rare Diseases report prepared by AIFD&IQVIA was evaluated. |
|
26/05/2022 |
TITCK & GHI (GSS) |
Governmental Workshop |
Mutual evaluations were made on Prioritization Processes, Pricing and Exchange Rate Policies, Parallel Export issues. |
|
22/06/2022 |
TITCK Vice Presidency of Audit Services |
Dr. Ecz. Sevil Azak Sungur |
Evaluations were made within the scope of on-site inspection plans, Reliance Implementation in the Regulation on Manufacturers of Medicinal Products for Human Use, and GMP Prioritization.. |
|
29/06/2022 |
TİSD, İEİS |
Dernek ve Sendika temsilcileri |
Evaluations were made within the scope of the Good Distribution Practices Guidelines. |
|
01/07/2022 |
TITCK Vice Presidency of Pharmaceuticals and Pharmacy, TITCK Department of Pharmaceutical Licensing |
Dr. Asım Hocaoğlu, Uzm. Ecz. Handan Öztunca |
Evaluations were made within the scope of our Sector Problems Letter sent to TITCK. |
|
03/08/2022 |
TITCK Vice Presidency of Pharmaceuticals and Pharmacy |
Dr. Asım Hocaoğlu |
Evaluations were made on prioritization, licensing processes, expected legislation, e-CTD and communication. |
|
21/09/2022 |
TITCK Department of Pharmaceutical Licensing |
Uzm. Ecz. Handan Öztunca |
Evaluations were made on current issues such as e-CTD transition plan, prioritization processes, Reliance practices approach, Frequently Asked Questions document, appointments with TITCK departments. |
|
17/10/2022 |
TITCK Presidency |
Dr. Asım Hocaoğlu |
Assessments were made on the prioritization process and other sectoral issues. |
|
17/10/2022 |
Vice Presidency of Ministry of Health |
Doç. Dr. Tolga Tolunay |
Assessments were made on the prioritization process and other sectoral issues. |
|
08/11/2022 |
TITCK Presidency, TITCK Dept. of Pharmaceutical Licensing |
Dr. Asım Hocaoğlu, Uzm. Ecz. Handan Öztunca |
Evaluations were made on the Prioritization Guidelines, the current situation regarding the elimination of analysis processes for licensing purposes, WHO Audit process, TITCK & AIFD Consultation Meeting, participation to DIA 2023 |
|
08/11/2022 |
TITCK Vice Presidency of Audit Services TITCK Vice Presidency of Pharmaceuticals and Pharmacy |
Dr. Ecz. Sevil Azak Sungur, Kim. Müh Filiz Ozul |
Assessments were made on Mutual Recognition Agreements (MRAs), MRAs that TITCK can conclude with PIC/s Member Countries, On-Site Inspection Plans and Certificate Periods, TITCK & AIFD Consultative Meeting, List of inspected facilities, Reliance Guideline for Good Manufacturing Practice Assessments. |
|
21/11/2022 |
TITCK Dept. of Pharmaceutical Licensing |
Uzm. Ecz. Handan Öztunca |
Evaluations were made on the Licensing Regulation and current issues. |
Submitted Official Letters
|
DATE |
INSTITUTION |
SUBJECT |
|
28/01/2022 |
TITCK Vice Presidency of Pharmaceuticals and Pharmacy |
Information and country examples within the scope of reliance in regulatory and supervisory activities |
|
31/01/2021 |
TITCK Licensing Coordination Unit |
Frequently Asked Questions document complied from questions of AIFD members |
|
08/02/2022 |
TITCK Vice Presidency of Pharmaceuticals and Pharmacy |
AIFD agenda items for TITCK Consultancy Meeting |
|
14/03/2022 |
TITCK Vice Presidency of Pharmaceuticals and Pharmacy |
AIFD input for the Draft Good Distribution Practices Guidelines |
|
29/03/2022 |
TITCK Information Systems Department |
ITS database (Pharmaceutical Track and Trace System) change time extension request (joint letter of 4 associations) |
|
08/04/2022 |
TITCK Herbal and Dietary Supplement Products Dept |
AIFD comments on the Draft Regulation on the Use of Health Claims |
|
19/04/2022 |
TITCK Deputy Head of Drug Inspection Dept.
|
AIFD comments on the Draft Regulation on Pharmaceutical Traders and Products Stored in Pharmaceutical Traders |
|
14/06/2022 |
TITCK Dept. of Pharmaceutical Licensing |
AIFD Questions under the Diversification Guidance |
|
15/06/2022 |
TITCK Dept. of Pharmaceutical Licensing |
Joint letter of associations on sector issues |
|
15/06/2022 |
General Directorate of Health Services, (SHGM) Dept. of Autism, Mental Special Needs and Rare Diseases |
AIFD Comments on the Draft Document on Rare Diseases Health Strategy |
|
07/07/2022 |
TITCK Dept. of Pharmaceutical Licensing |
Extension of Nitrozamin timeline (joint letter of 4 industry associations) |
|
18/07/2022 |
TITCK Deputy Head of Drug Inspection Dept. |
AIFD views for Guidance on Applications for GMP Audits of Overseas Production Facilities |
|
02/08/2022 |
TITCK Deputy Head of Drug Inspection Dept. |
Frequently Asked Questions compiled from AIFD member companies |
|
22/11/2022 |
TITCK Dept. of Pharmaceutical Licensing |
Problems experienced by AIFD member companies within the scope of Variation Application, Evaluation and related EBS (electronic application) processes, and suggestions for solutions to these problems |
|
02/12/2022 |
TITCK Deputy Head of Drug Inspection Dept.
|
AIFD views for the revised Guidance on Applications for GMP Audits of Overseas Production Facilities |
|
16/12/2022 |
General Directorate of Health Services, (SHGM) Dept. of Autism, Mental Special Needs and Rare Diseases |
About the workshop to be held within the scope of Rare Diseases Health Strategy Document and Action Plan |
During 2022, the Meetings/Symposiums/Congresses attended online or physically by AIFD professionals are presented below:
|
DATE |
NAME OF THE MEETING/SYMPOSIUM/CONGRESS |
|
20/01/2022 |
İstinye Uni. Pharmacy Faculty external stakeholders meeting |
|
15/02/2022 |
TITCK Sector Consultative Meeting |
|
23/02/2022 |
IPTS (International Pharmaceutical Technology Symposium) 2022 |
|
28/02/2022 |
Health Services General Directorate Rare Diseases Awareness Day Symposium |
|
24/02/2022 |
Hacettepe Uni. Pharmacy Faculty external stakeholders meeting |
|
10/03/2022 |
8th Hospital and Institution Pharmacists Congress |
|
28/03-01/04/2022 |
Drug Information Association (DIA) EUROPE Meeting |
|
28/05/2022 |
Metabolic Diseases and Nutrition Congress |
|
02/06/2022 |
TÜSEB Future Health Technologies Summit |
|
20-21/10/2022 |
Bioexpo 2022 |
|
15/12/2022 |
(WHO 10th Annual Meeting on Collaborative Registration Procedure) |
|
16/12/2022 |
6th Health Economics Congress Health Economics and Policies Association (SEPD ) |
|
22/12/2022 |
Rare Diseases Future, Centers of Excellence Symposium |
AIFD actively participated in the Licensing and Biosimilar Subcommittees of the Pharmaceutical Industry Council of the Union of Chambers and Commodity Exchanges of Turkey (TOBB). With the cooperation of participants from associations, trade unions and companies representing the sector in these committees, content was prepared in the meetings shown in the list below, to be shared with TITCK within the scope of the evaluations made on the legislation as well as the needs of the sector. Presentations on the relevant content were made at the Turkish Pharmaceutical Industry Council held on December 26, 2022.
|
DATE |
MEETING |
|
18/10/2022 |
TOBB Pharmaceutical Sector Council Licensing Sub-Committee Meeting |
|
25/10/2022 |
TOBB Pharmaceutical Sector Council Biosimilars Subcommittee Meeting |
|
08/11/2022 |
TOBB Pharmaceutical Sector Council Licensing Sub-Committee Meeting |
|
15/11/2022 |
TOBB Pharmaceutical Sector Council Licensing Sub-Committee Meeting |
|
22/11/2022 |
TOBB Pharmaceutical Sector Council Biosimilars Subcommittee Meeting |

