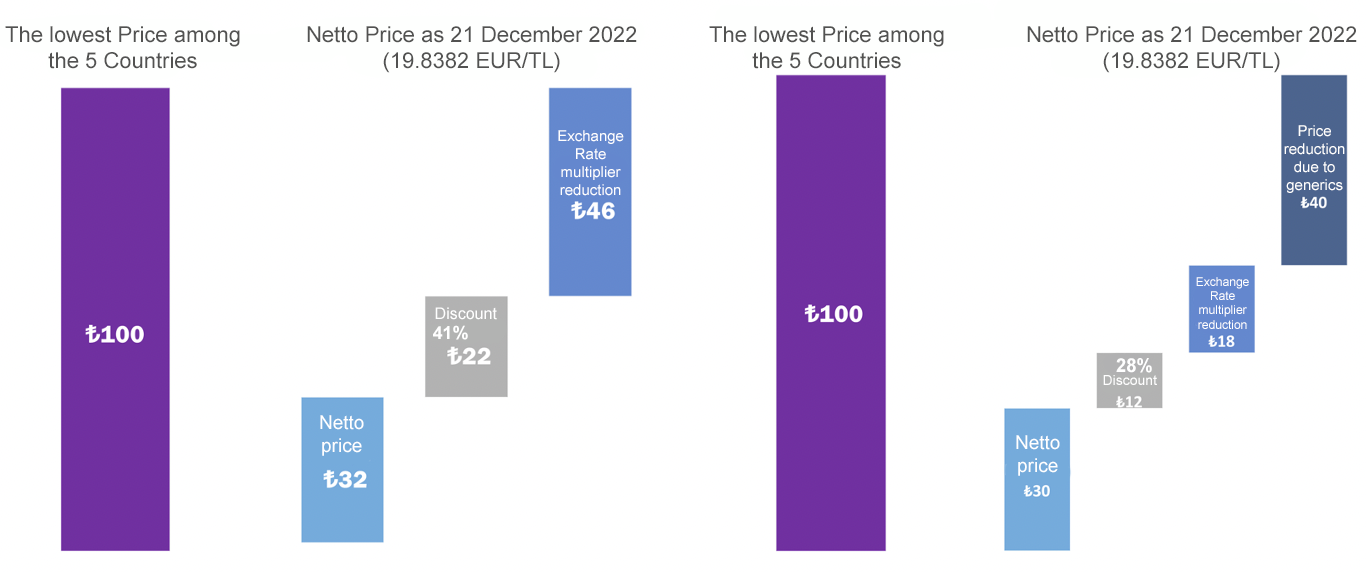
AIFD's solution proposals, outlined above, have been expressed in all meetings held in the public area, including in a large scale workshop organized in May 2022 with the participation of all competent public authorities (Ministry of Health, TİTCK, Ministry of Treasury and Finance, Presidency of Strategy and Budget, SSI/GSS).
In order to ensure that the legislative infrastructure of the proposals on pricing is also provided, AIFD has also prepared a solid structural study in 2022 for updating the "Decision on the Pricing of Medicinal Products for Human Use" and the "Communiqué on the Pricing of Medicinal Products for Human Use", and will start discussions with the TITCKin the coming period.
On the other hand, within the framework of the calendar stipulated in Article 10 of the Communiqué on the Pricing of Medicinal Products for Human Use in 2022, the First Interim list was published on September 30 as a result of the real source price change application evaluations, and when the said list was examined, it was seen that, unlike the practices of the Agency in previous years, products that are predominantly hospital products in Greece, one of the countries accepted as a source country by our country within the scope of Article 4 of the Communiqué, were erroneously identified as source products and hospital product prices were taken into account in the real source price calculation. AIFD immediately initiated a legal evaluation process and requested the correction of the practice through high-level meetings. As part of our efforts, we contacted the Greek pharmaceutical association SFEE and received official opinions on the system in that country. As a result of the evaluations made, an official letter containing AIFD's legal assessment and solution request was sent to TITCKon October 25, 2022, stating that the practice of taking hospital products into account in the calculation of the real source price is contrary to the letter and spirit of the communiqué and that hospital products should not be used in the calculation of the real source price. As a result of AIFD's intensive efforts, this practice was abandoned and the necessary legislative amendment was made by the institution to resolve the issue. In this context, the "Decision on the Amendment of the Decision on the Pricing of Medicinal Products for Human Use" was published in the Official Gazette dated November 9, 2022 and numbered 32008, and Article 2 of the "Decision on the Pricing of Medicinal Products for Human Use", which was put into effect with the Council of Ministers Decision dated February 6, 2017 and numbered 2017/9901, was amended as follows: " 12) Discount practices that cause temporary price changes in the country/countries where the product is offered for sale, special practices regarding product classification and special taxation practices are not taken into account in the calculation of the real source price. Products that do not have retail sales in the country/countries where they are offered for sale and are only available through hospitals are not taken into account in the calculation of the real source price of products with retail sales in our country. Applicant companies shall submit the documents certifying this issue to the Agency with the application ."
Legislation Developments during 2022 concerning Pricing:
|
6/01/2022 |
On the Official Gazette dated January 6, 2022 and numbered 31711, the "Decision on Amendment to the Decision on Pricing of Medicinal Products for Human Use" was published. The following temporary article was added: "From the final increase rates formed on product basis within the framework of the decisions taken by the Commission until July 10, 2015; After July 10, 2015, these final increase rates for products for which no new decision has been taken after July 10, 2015, and for products for which the final increase rate has been reduced by new decisions taken by the Commission after July 10, 2015, the newly determined final increase rates will continue to be exempted from the offsetting process in the third paragraph of Article 3. " |
|
14/02/2022 |
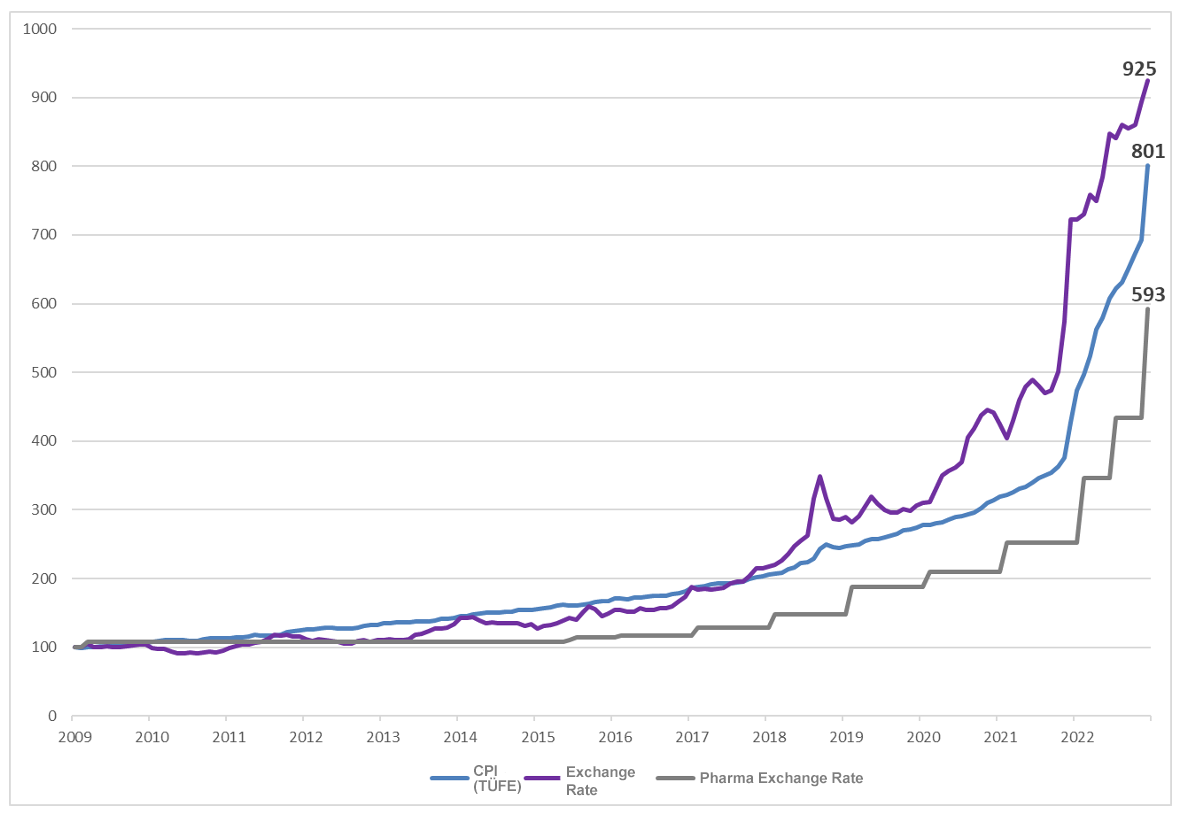
On February 14, 2022, the TITCKAnnouncement and the decisions of the Price Evaluation Commission (FDK) were published. With the FDK decisions, the value of one Euro used in the pricing of Medicinal Products for Human Use for the year 2022 was increased by 37.43% from 4.5786 TL to 6.2925 TL. |
|
19/04/2022 |
Within the scope of the "Decision Amending the Decision on the Pricing of Medicinal Products for Human Use" published in the Official Gazette dated 19 April 2022 and numbered 31814; the following statements were added: "After the products included in the foreign drug list are licensed in our country, the actual source prices are determined according to the provisions of this Decision and the Communiqué on the Pricing of Medicinal Products for Human Use. However, the warehouse sales prices of the products cannot exceed the current exchange rate equivalent of the price in the Foreign Drug Price List (Annex 4/C) attached to the Social Security Institution Health Implementation Communiqué." |
|
22/04/2022 |
With the Detailed Drug Price List of the TITCKdated April 22, 2022 and the Price Evaluation Commission Decisions dated April 11-19, 2022, various adjustments were made, particularly for drugs that have received a DSF increase, and these adjustments entered into force on April 26, 2022. |
|
9/05/2022 |
The Health Industries Coordination and Monitoring Unit of the Economic Assessments and Pharmaceutical Supply Management Department of TITCKpublished a report on the general situation of the Turkish pharmaceutical market. |
|
8/07/2022 |
With the "Provisional Article 5-(1) of the Decree Amending the Decree on the Pricing of Medicinal Products for Human Use (Decree Number: 5800) published in the Official Gazette dated July 8, 2022 and numbered 31890, the provision "1 (one) Euro value in Turkish Lira to be used in the pricing of Medicinal Products for Human Use; shall be increased by 25% in July 2022" was published. In the price list published on the official website of TITCKon July 8, 2022, the value of 1 (one) Euro used in the pricing of Medicinal Products for Human Use for July 2022 was 7.8656 TL. In the same price list, it is stated that the validity date of the application is July 9, 2022. |
|
5/08/2022 |
With the TİTCK's announcement dated August 5, 2022, it was stated that in 2020 and 2021, when apostilled or consulate-approved documents could not be submitted in the processes of pricing of medicinal products for human use and notification of real source price changes due to the COVID-19 pandemic, the relevant documents without apostille or consulate approval were accepted with the commitment of the company during the pandemic; and in the processes of notification of real source price changes in 2022, the documents with a company commitment that the Apostille or Consulate-approved document will be submitted later will not be accepted. For this reason, in the applications for the period of 2022; it has been announced that the original approved documents must be submitted to the Agency on the application dates specified in the fourth paragraph of Article 10 of the Communiqué on the Pricing of Medicinal Products for Human Use. |
|
29/08/2022 |
The "Guideline on Import Applications and Authorization for Placing on the Market" was published by the Economic Evaluations and Pharmaceutical Supply Management Department of the TITCKand entered into force on the same date. |
|
12/10/2022 |
On October 12, 2022, "Decision Amending the Decision on the Pricing of Medicinal Products for Human Use" was published in the Official Gazette dated October 12, 2022 and numbered 31981. With the relevant decision, warehouse and pharmacist profit rates have been updated. |
|
9/11/2022 |
"Decision on the Amendment of the Decision on the Pricing of Medicinal Products for Human Use" was published in the Official Gazette dated November 9, 2022 and numbered 32008, and Article 2 of the "Decision on the Pricing of Medicinal Products for Human Use", which was put into effect with the Council of Ministers Decision dated February 6, 2017 and numbered 2017/9901, was amended as follows: "12) Discount practices that cause temporary price changes in the country/countries where the product is offered for sale, special practices regarding product classification and special taxation practices are not taken into account in the calculation of the real source price. Products that do not have retail sales in the country/countries where they are offered for sale and are only available through hospitals are not taken into account in the calculation of the real source price of products with retail sales in our country. Applicant companies shall submit the documents certifying this issue to the Agency with the application." paragraph has been added. . |
|
14/12/2022 |
With the "Decision Amending the Decision on the Pricing of Medicinal Products for Human Use" published in the Official Gazette dated December 14, 2022 and numbered 32043, the Euro value to be used in the pricing of pharmaceuticals was increased by 36.77% from 7.8656 TL to 10.7577 TL, effective from December 15, 2022, and it was stated that no offsetting will be made in the FDK rates while applying this increase, the determined Euro value will continue to be applied for 2023 and no new Euro update will be made in February 2023. The threshold values mentioned in the tenth paragraph of Article 2 of the Decree on the Pricing of Medicinal Products for Human Use were also updated as TR 37.10 for price-protected products and TR 19.39 for other products. |
|
15/12/2022 |
The TITCKDepartment of Economic Evaluations and Pharmaceutical Supply Management published an announcement on the "Cancellation of the Decision on the Extension of the Approval Periods for the Use of Off-Indication/International Medicinal Products". |
2022 Important meetings, visits and correspondence on Pricing organized by AIFD:
|
7-9/01/2022 |
AIFD participated to the Pharmaceutical Sector Future Research Workshop organized in Sapanca by IKMIB (Istanbul Chemicals and Chemical Products Exporters' Association) . |
|
19-20/01/2022 |
Within the scope of the meetings held in Ankara, AIFD Chair of the Health Economics SMC met Osman Nuri Erdem, Director General of Plans and Programs at the Strategy and Budget Directorate, Dr. İbrahim Muaz Yaradılmış, Head of Economic Assessments and Pharmaceutical Supply Management Department at TITCK, Dr. İrfan Tuncay Alkan, Head of Pharmaceuticals Department at GSS, Ahmet Oğuz Sarıca, Advisor to the Minister of Health, and Dr. Refik ALTUN, Head of Health Resource Management Department at GSS. During the meetings, mutual views were exchanged on the future of the pharmaceutical sector with the impact of the developments in our country's economy. |
|
21/02/2022 |
During the official visit to Hakkı KARABÖRKLÜ, General Director of Product Safety and Inspection of the Ministry of Trade, evaluations were made on current sectoral issues and problems, especially parallel trade. |
|
23-27/03/2022 |
AIFD participated to the Joint Solution in Health Meeting organized by OHSAD. (Association of Private Hospitals and Health Organisations) . |
|
6/04/2022 |
The 2021 results of the WAIT Survey, which presents the rate of access to innovative products in Turkey in comparison with European countries, were announced. |
|
14/04/2022 |
During the visit to the office of Deputy Minister of Health Assoc. Prof. Dr. Tolga Tolunay with a delegation led by AIFD Chairman Dr. Mete Hüsemoğlu, the priority issues and problems on the agenda of our sector, especially the pharmaceutical exchange rate, were conveyed in detail and mutual evaluations were made for their solutions.
|
|
28/04/2022 |
A delegation led by Dr. Mete Hüsemoğlu, Chairman of the AIFD Board of Directors, met with Assoc. Prof. Dr. Tolga KARAKAN, President of TITCK, to discuss the planning of a workshop to be held with the participation of AIFD, TITCK and members of the Price Evaluation Commission. |
|
28/04/2022 |
With the participation of the Health Economy Policies SMC Management team, we met with Ahmet Oğuz Sarıca, Advisor to the Minister of Health, and Erkan Tek, Deputy Director General of Public Capital Institutions and Enterprises at the Ministry of Treasury and Finance, and discussed in detail the priority issues and problems on the agenda of our sector, especially the drug exchange rate problem, and made mutual evaluations for their solutions. |
|
10/05/2022 |
In the official letter sent to TITCKwith the joint signature of AIFD, İEİS, SURDER and TİSD, it was emphasized that the issue of parallel trade in pharmaceuticals should not be evaluated like re-export in other sectors, referring to the fact that the price of pharmaceuticals, unlike other products, is not determined under free market conditions, and that the problems created by parallel trade threaten the security of pharmaceutical supply in our country, especially for cancer and chronic diseases, risks to the supply chain security and integrity of products, challenges to the global operations of global companies, and threats to the efforts of our manufacturer-exporter companies, particularly emphasized for local companies, to establish themselves in foreign markets, and in this context, the pharmaceutical industry stakeholder organizations requested "a legislative amendment to include a declaration from the legal marketing authorization holder in the mandatory documents accompanying exports" as a solution to the problem. |
|
26/05/2022 FOTO |
A comprehensive workshop was organized with the participation of all competent public authorities (Ministry of Health, TİTCK, Ministry of Treasury and Finance, Presidency of Strategy and Budget, SSI/GSS) and the priority issues and problems on the agenda of our sector, particularly pricing-related problems, parallel trade, prioritization and foreign drug list, were discussed in detail. Following the meeting, technical work was initiated with the public sector to follow up on the issues.
|
|
15/06/2022 |
We participated in the Pharmaceutical Industry Technical Committee (İLAÇTEK) Meeting organized by the Ministry of Industry and Technology and made a presentation on the current situation of the pharmaceutical industry, exchange rate-related problems, access to innovative medicines in our country, parallel trade and the importance of clinical trials. |
|
23/06/2022 |
A delegation headed by Dr. Mete Hüsemoğlu, Chairman of the Board of Directors of AIFD, participated in the meeting of the Presidential Health and Food Policies Board, and a comprehensive presentation was made to the aforementioned Board, including our expectations regarding the drug exchange rate, especially the second exchange rate update in 2022, and our sectoral problems and solution suggestions.
|
|
30/06/2022 |
Within the scope of the Competition Authority's "Pharmaceuticals Sector Survey", an online information meeting was held with the said institution. |
|
9/09/2022 |
AIFD participated to the 28th Vision Meeting organized by TÜSAP. (Türkiye Sağlık Platformu) |
|
13/09/2022 |
Within the scope of the AIFD Health Economics Policies SMC meeting, Ekotürk Research Manager Cenk Akyoldaş provided information about the Medium Term Program (MTP -OVP/2023-2025) announced on September 5, 2022. |
|
13/09/2022 |
We attended the TOBB Pharmaceutical Industry Council Meeting, where the periodical pharmaceutical euro exchange rate, pricing legislation, reimbursement legislation and SSI practices, licensing and export issues were discussed in detail. In addition, it was decided that the chairmen and members of the subcommittees of the TOBB Pharmaceutical Assembly would be selected and start working as soon as possible. |
|
14/09/2022 |
Participation was ensured in the İKMİB Pharmaceutical Sector Committee Meeting held in Istanbul under the chairmanship of İKMİB Chairman Adil Pelister. |
|
20/09/2022 |
With the participation of the general secretaries of the Association of Research-Based Pharmaceutical Companies, the Pharmaceutical Industry Employers' Association and the Pharmaceutical Industry Association, which are members of the TOBB Pharmaceutical Industry Council, the office of Mehmet Kürşat Derici, Vice President of TİTCK, was visited and the issue of parallel trade and the demand for a solution was expressed.. |
|
30/09/2022 |
On September 30, 2022, the First Interim list was published as a result of the real source price change application evaluations within the framework of the calendar stipulated in Article 10 of the "Communiqué on the Pricing of Medicinal Products for Human Use" (Communiqué) published in the Official Gazette dated September 29, 2022 and numbered 30195, and it was discovered that product prices of hospital product status mainly from Greece were taken into account in the real source price calculation, contrary to the practices of the Agency in previous years, Greece being one of the countries accepted as a source country by Türkiye within the scope of Article 4 of the Communiqué. AIFD initiated a legal evaluation process. . |
|
5/10/2022 |
TOBB Pharmaceutical Council Price and Reimbursement Sub-Committee Meeting was attended. |
|
12/10/2022 |
In the 2022 Price Change Period First Interim List, AIFD met with Assoc. Prof. Dr. Mehmet Kürşat Derici, Vice President of TİTCK, regarding the use of hospital product prices in the real source price calculation and AIFD's evaluations were conveyed. |
|
13/10/2022 |
AIFD took part in IQVIA Client Day Meeting held in Istanbul. |
|
17/10/2022 |
A delegation led by Dr. Mete Hüsemoğlu, Chairman of the AIFD Board of Directors, paid official visits to Deputy Minister of Health Mr. Tolga Tolunay and TITCKPresident Dr. Asım Hocaoğlu to convey our evaluations regarding the use of hospital product prices in the real source price calculation in the 2022 Price Change Period First Interim List. During these meetings, prioritization and other sectoral issues were discussed, as well as the inclusion of hospital products in the real source price calculation. |
|
18/10/2022 |
Regarding the consideration of hospital products in the real source price calculation, the official opinion on the system of the country was obtained by contacting the Greek pharmaceutical association SFEE. |
|
25/10/2022 |
An official letter containing AIFD's legal assessment and request for a solution regarding the inclusion of hospital products in the calculation of real source prices, which is contrary to the letter and spirit of the communiqué, and that hospital products should not be used in the calculation of real source prices, was sent to TİTCK. |
|
27/10/2022 |
A meeting was held with Kürşat Derici, Vice President of TİTCK, regarding the practice of taking hospital products into account in the calculation of real source prices. |
|
5/11/2022 25/11/2022 |
In order to share the results of the WAIT Survey, which reveals the rate of access to innovative products in Turkey in comparison with European countries, with relevant stakeholders and to raise awareness on this issue, presentations were made at the 48th National Hematology Congress held on November 2-5, 2022 and at the HIV Congress held on November 24-27.
|
|
9/11/2022 |
TOBB-Price and Reimbursement Sub-Committee Meeting was attended. |
|
29/11/2022 |
AIFD Board of Directors participated in the pharmaceutical sector meeting organized under the chairmanship of Mahmut Gürcan, Deputy Minister of Treasury and Finance. At the aforementioned meeting, the problems of our sector, especially the drug exchange rate, and detailed solution proposals were conveyed. |
|
15/12/2022 |
During the meeting with Dr. İbrahim Muaz Yaradılmış, Head of Economic Assessments and Pharmaceutical Supply Department at TITCK, the results of EFPIA's WAIT Survey were evaluated. |
|
16-17/12/2022 |
AIFD participated in the VIth Health Economics Congress titled "New Approaches to Licensing, Pricing and Access to Innovative Therapies" held in Ankara. |
|
16/12/2022 |
A delegation led by Dr. Mete Hüsemoğlu, Chairman of the Board of Directors of AIFD, participated in the official meeting of the Deputy Minister of Treasury and Finance, Mr. Mahmut Gürcan, with industry representatives.
|
|
16/12/2022 |
A delegation headed by Dr. Mete Hüsemoğlu, Chairman of the Board of Directors of AIFD, participated in the high-level meeting of our Minister of Health, Mr. Fahrettin Koca, with representatives of the pharmaceutical sector, and the reflections of the drug exchange rate update on the sector and the additional steps to be taken were discussed at the meeting.
|
|
21/12/2022 |
AIFD participated in the 11th Meeting of the Pharmaceutical Industry Technical Committee (İLAÇTEK) organized by the Ministry of Industry and Technology. |
|
22/12/2022 |
As part of the 12th Development Plan preparations, AIFD participated in the first-round meeting of the Working Group on Pharmaceuticals in the Health System held in Ankara. . |
|
23/12/2022 |
As part of the 12th Development Plan preparations, AIFD participated in the first round meeting of the Specialized Commission on Transformation in Health Industries held in Ankara. |
|
26/12/2022 |
The activities carried out by the Subcommittees on "Pricing and Reimbursement", "Licensing", "Biotechnology and Biosimilar Medicines", "R&D, Localization and Manufacturing" and "Healthcare Products" were presented at the TOBB Pharmaceutical Industry Council Meeting. |
|
31/12/2022 |
"Communiqué Amending the Communiqué on the Pricing of Medicinal Products for Human Use" was published in the Official Gazette dated December 31, 2022 and numbered 32060, and within the scope of the relevant communiqué amendment, the method of determining the real source price of allergy products was detailed in separate paragraphs in the communiqué, and the real source price determination and real source price change sections of the applications made with the cost card were amended. Amendments have been made to the "Exceptional Real Source Price Increases" section of the Communiqué. |

