Facts & Figures
Reimbursement Survey
Findings are as followed:
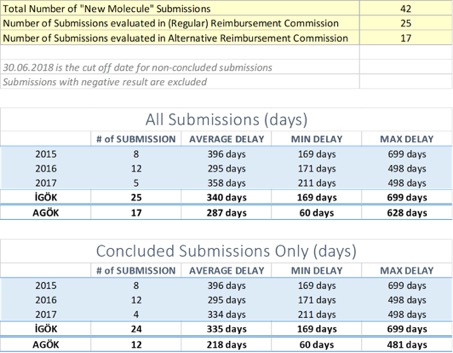
Average Delay for New Molecules is 318 days/45 weeks which includes not-concluded submissions and alternative reimbursement process (which is alone 287 days/41 weeks).
For regular reimbursement process, average delay in 2017 is shorter than 2015 but longer than 2016. Average delays from 2015 to 2017 are 396 days/57 weeks, 295 days/42 weeks, 358 days/51 weeks respectively.
Nearly half of the new molecules accessed to the market through “4/C” channel at first. After submission to positive list, average delay for those molecules is shorter than other submissions: 279 days/39 weeks vs 348 days/49 weeks.
Two third of the new molecules (22 out of 35) are asked additional price decrease/discount for entering positive list.
Submissions that are moved from ‘regular’ reimbursement commission to ‘alternative’ have longer delays than others: 407 days/58 weeks vs 298 days/42 weeks
