Turkey aims to be regional leader in the area of clinical research
17.09.2021
The Association of Research-Based Pharmaceutical Companies (AIFD) and the European Federation of Pharmaceutical Industry and Associations (EFPIA) jointly organized a webinar titled “Building an attractive environment for clinical research in Turkey.” The webinar discussed the developments recorded in clinical research in Turkey and other countries. In the event, the Deputy Minister of Health of the Republic of Turkey Assoc. Prof. Mr. Tolga Tolunay, emphasized Turkey’s commitment to clinical research and reported the efforts to make Turkey a preferred environment for clinical research.
The webinar “Building an attractive environment for clinical research in Turkey”, jointly organized by the Association of Research-Based Pharmaceutical Companies (AIFD) and the European Federation of Pharmaceutical Industry and Associations (EFPIA), took place on Tuesday, September 14.
In his opening speech, EFPIA Director of International Relations, Dr. Koen Berden said, “With more than 5000 clinical research starting in 2020 alone, we are entering a new era that will have a profound impact on healthcare around the world. It is possible to encounter promising innovative treatments in many fields such as cell and gene therapies and the treatment of Alzheimer’s. Many countries are struggling to unleash the potential for clinical R&D studies that are needed for innovative medicines and offer much higher added value by putting more emphasis on production. Clinical R&D has far wider implications for society, the healthcare system, and patients; with regards to healthcare industry, there is an important potential to enable more clinical research to be conducted in Turkey.”
In his speech, Deputy Minister of Health of the Republic of Turkey, Assoc. Prof. Mr. Tolga Tolunay stated that “Pharmaceutical and medical device industry is among the prioritized industries in the development plan. The main purpose of this meta policy document is to increase Turkey’s competitiveness in the global market and to move our country to a higher position in the pharmaceutical value chain.” Tolunay added, “It is of great importance to develop the clinical research infrastructure in Turkey because it is one of the most important steps of pharmaceutical R&D.. It is one of our essential policies to ensure that our country becomes the leading country in the region in clinical research.” Tolunay emphasized that the Turkish Medicines and Medical Devices Agency (TITCK) maintains its regulatory and supervisory function in clinical research at international standards.
Tolunay continued his words as follows: “As of August 2021, the number of active researches in progress has reached 1027. 31 percent of the applications made between 2012 and 2020 are phase 3 studies, 24 percent observational studies, 20 percent bioavailability/bioequivalence studies, 15 percent phase 4, 9 percent phase 2 and 1 percent phase 1 research. We aim to increase the number of researches carried out and to double the number of phase 1 centers.”
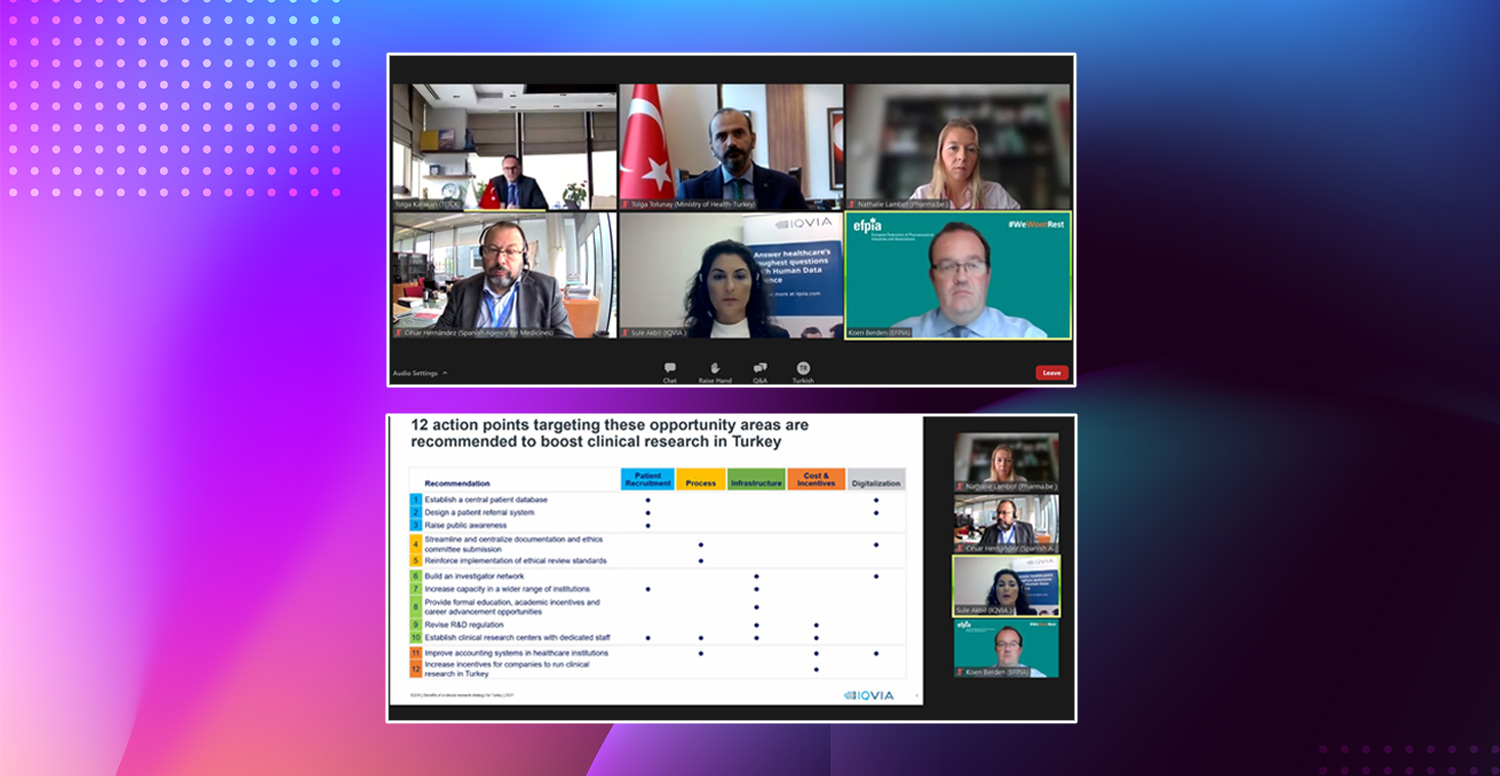
Tolunay stated that effective cooperation is maintained with relevant public institutions, industry representatives, NGOs and other stakeholders in order to achieve their goals as a country in the field of clinical research. He said, “Actions that need to be taken are implemented together with the stakeholders. An effective example of this collaboration and work is the report titled “Benefits of Clinical Research Strategy for Turkey, Roadmap for Innovation-Driven Growth” prepared with the support of AIFD, as well as the participation of public and industry representatives, academics and other relevant parties. The report examines the clinical research environment in the world and in our country, presents the global clinical research trends, and offers suggestions on increasing clinical research with the development of the clinical research infrastructure in our country. The report is an inspiring resource for all stakeholders on the way to our goal of becoming a regional leader. As an output of this report, a working group was formed with our public representatives, sector representatives, NGOs and academicians. This working group is active in many areas such as increasing the awareness of clinical research parties and the public on clinical research, increasing the number of phase 1 centers and increasing the awareness of our country on clinical research in the international platform.”
In her presentation, IQVIA Engagement Manager, Ms. Şule Sencer Akbil said, “The report titled ‘Benefits of Clinical Research Strategy for Turkey – Roadmap for Innovation-Driven Growth’ published by IQVIA with the contributions of EFPIA and AIFD, reveals the contributions of clinical studies to our country and the role of Turkey in achieving economic policy goals. The analysis carried out within the framework of the report have shown that there are many factors that make a country attractive in the field of international clinical research. Some of these factors such as patient recruitment, process, infrastructure and cost, and incentives, development opportunities were also observed for Turkey, and various suggestions have been presented to produce solutions in these opportunity areas. It is anticipated that most of these recommendations will contribute significantly to the increase in clinical research in our country and have positive impact on more than one area of development.
At the event, pharma.be Clinical Trials & Regulatory, Public Health Specialist Ms. Nathalie Lambot shared the Belgian example of creating a clinical research center, while the Spanish Medicines Agency (AEMPS) Director of the Human Medicines Department, Dr. César Hernandez García talked about their experience in creating a successful environment for clinical research in Spain.

