AUDITING & INSPECTION
The vision of AIFD, representing 35 global pharmaceutical companies operating in Turkey, is to “be a solution partner” of the government and of the healthcare sector in our country by "proposing innovative treatment options” to overcome the difficulties faced in the field of healthcare. One of our top priorities therefore is the Access to innovative medicines – that the patients in Turkey access innovative medicines in similar timeframes and conditions as in EU and USA.
According to the European Pharmaceutical Industries and Associations Federation (EFPIA), annual GMP / GDP inspections survey, Turkey comes after Russia, USA and Japan ranked fourth in 2019 among the countries with the highest number of offshore inspections.
EFPIA & AIFD Inspection Workshop
An online meeting was organized in cooperation with EFPIA & AIFD on May 8, 2020 to share with AIFD members the results of the survey of the inspection processes carried out by EFPIA. Dr. Stephan Rönninger, EFPIA Inspection Working Group member, gave a presentation to AIFD members on the 2019 GMP survey results, on Inspection Reliance Process and Mutual Recognition approaches.
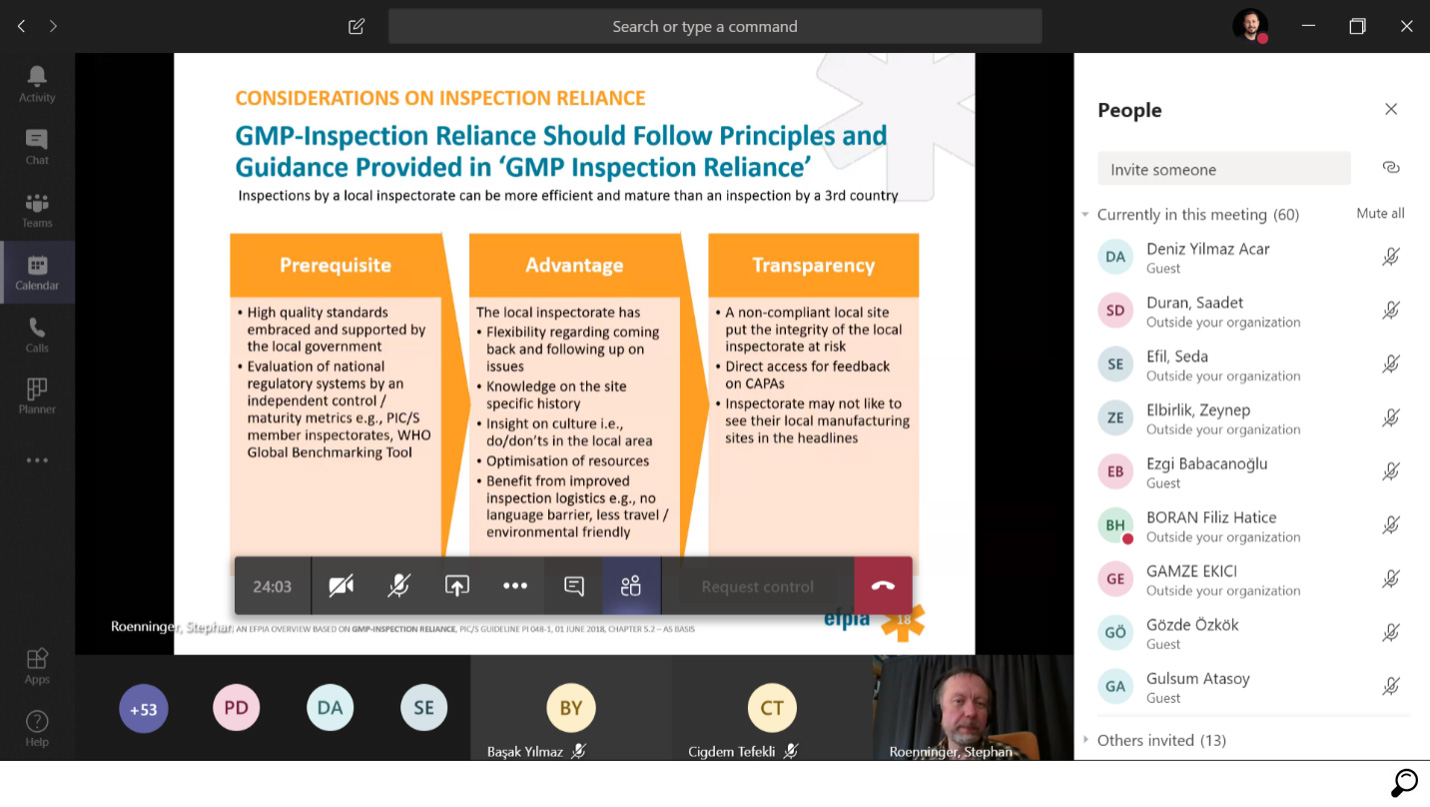 Mutual Recognition of GMP-Inspection Processes
Mutual Recognition of GMP-Inspection Processes
There is no doubt that an effective inspection system established by authorities to ensure the quality of the medicines and the confidence of patients in the drugs they use is critically important.
Today, global approaches are developed and strategic partnerships are established between countries in order to utilise the inspection capacities in an optimal way. This allows patients to rely on the quality, safety and efficacy of all medicines regardless of where they are produced.
As the latest example of this approach; The United States Food and Drug Administration (FDA) has cooperated with the European Medicines Agency (EMA) to reduce the audit workload and aimed to use its resources for GMP audits in developing countries that it considers potentially more risky for public health.
In this context, FDA has made mutual recognition agreements for GMP inspections with a total of 28 Health Authorities since 1 November 2017.
This approach is an important milestone towards closer cooperation to improve the use of available resources to protect the quality and safety of medicines. After a four years long devoted efforts of evaluation and inspection process, TITCK was accepted as a member of PIC/S (Pharmaceutical Inspection Co-operation Scheme) on September 12, 2017, and as of January 1, 2018, it has been included among the authorities whose audits would be accepted all over the world. Thus, the quality and safety of medicines manufactured in Turkey are formally acknowledged and the technical barriers they were facing to reach world markets were eliminated.
Negotiations for mutual recognition agreements with other countries after TITCK's PIC/S membership are reported to be ongoing, though there is no concluded agreement. After PIC/S membership, a similar approach could be taken by our country to conclude mutual recognition agreements, which will pave the way for Turkey to become a competitive player in the global market.
The annual GMP inspection survey among AIFD members was initiated in the second quarter of 2020, and the performance of new processes was measured. Current results were shared with our members in the third quarter of the year. According to the data of the 2020 survey study conducted by AIFD among its members covering the period between 01.01.2018 and 01.06.2020;
- Median time for high priority products (GMP ‐ 1) in GMP inspections last 309 days.
- In the 2020 Survey, there were three products in the on-site inspection category for new license GMP-2 priority products. Therefore, the median could not be calculated.
- In cases where GMP-3 category products are not incorporated to an existing audit, the timeframe is very long since no audit is carried out.




