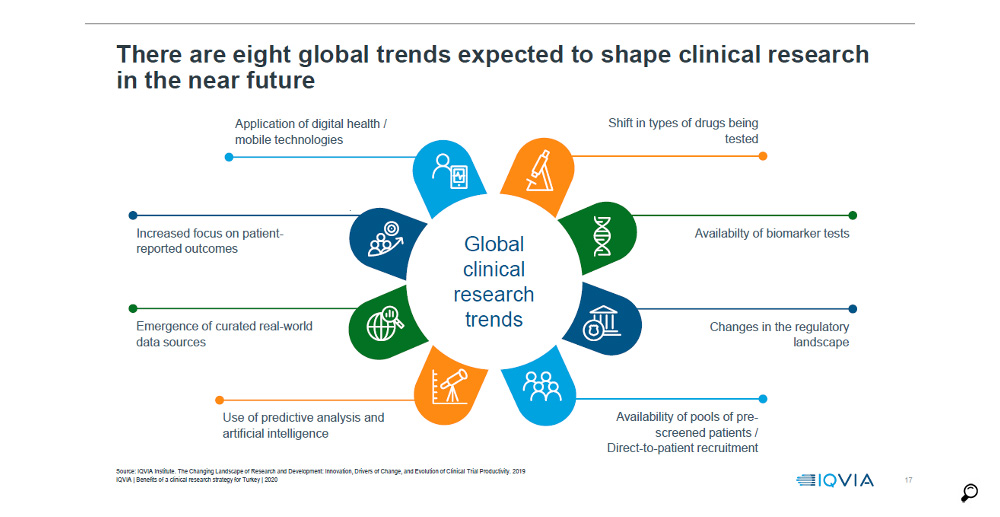
When we analyse the industry-sponsored clinical research in Turkey in the course of the last 10 years, it is observed that a leap occurred after 2013 with the implementation of the new measures by TITCK; the number of clinical trials increased from the 400 level to stablise at the 500 level. The number of clinical studies remained flat at that level during the last 5 years. Unless a breakthrough similar or even greater than that seen in 2013 is made, it seems unlikely to achieve the ambitious targets stated in the 10th and 11th Development Plans.
In order to reach the 11th Development Plan clinical studies target to be the leading country in the region in clinical trials, due to the multi-stakeholder nature of the clinical trials, all relevant agencies and parties must work in tandem towards the common goal simultaneously.
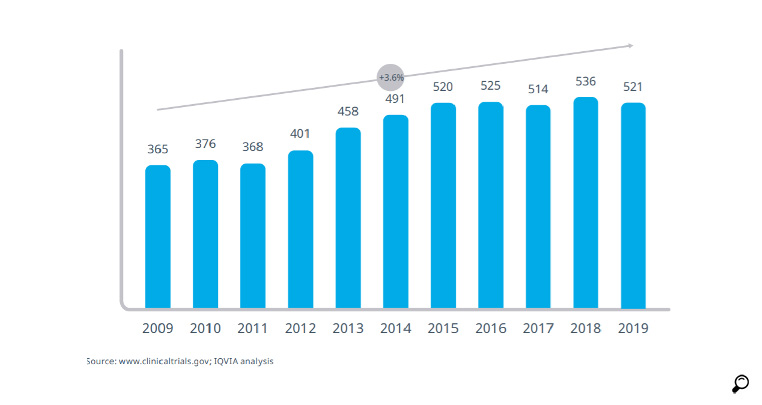
Various activities were carried out to double the number of ongoing 521 industry-supported clinical studies as of 2019 and to be among the top 15 countries in the world. As AIFD, in 2017 we held various workshops; in April 2017, with the participation of the public authorities and the pharma sector, we had an in-depth discussion of problems and solution suggestions in the 'Clinical Studies Roadmap' workshops with the participation of expert academics and AIFD representatives.
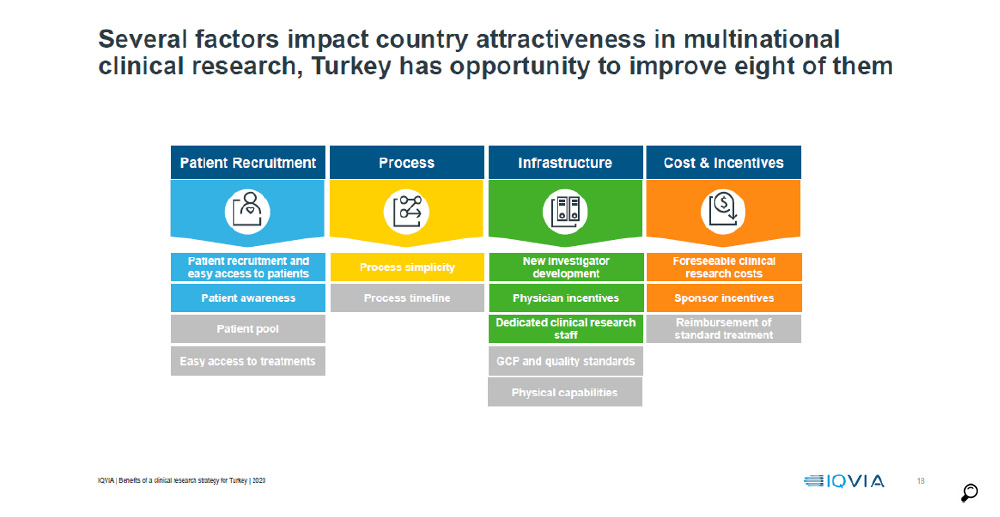
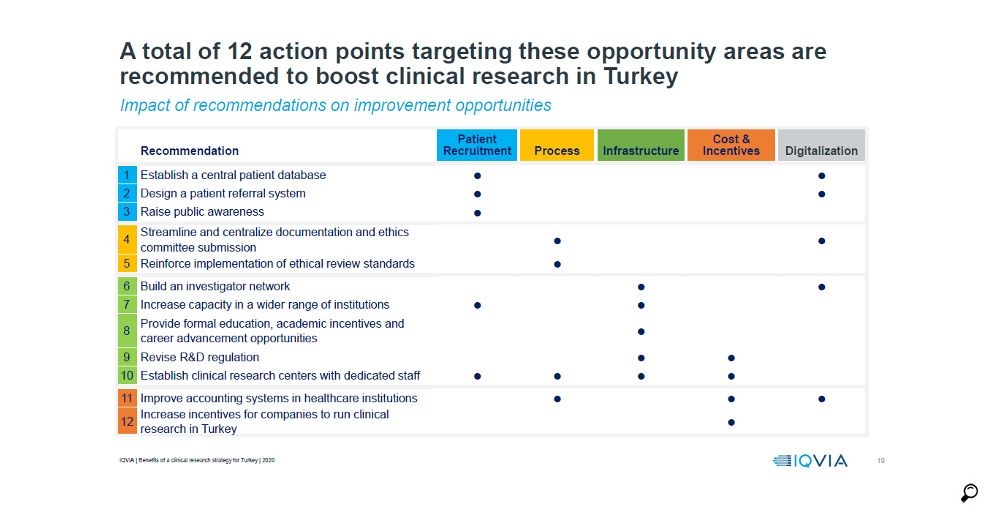
Finally, IQVIA prepared a report encompassing all previous studies, consolidating them in a wider perspective and with deeper insight, the "Innovation-Based Benefits Clinical Research Strategy for Growth Roadmap for Turkey" where the following recommendations have been developed: