There are several secondary detrimental effects of the seriously low pharmaceutical prices in Turkey, one of which is the role Turkey plays as a price reference for other countries which negatively affects the reimbursement prices in these countries; the second is the unregulated re-export of the products imported for and provided to the Turkish market.
Although the Euro reference prices are announced in the open-access price list of the Ministry of Health, after the recent exchange rate fluctuations, foreign health authorities started requesting also the Turkish Lira prices of the medicines. A recent case emerged towards the end of 2019, when the Chinese government has included Turkey in the reference countries for reimbursement calculations. As the price levels in Turkey also affects the reimbursement levels in other countries, pharmaceutical companies are becoming more reluctant to launch their new innovative molecules in Turkey
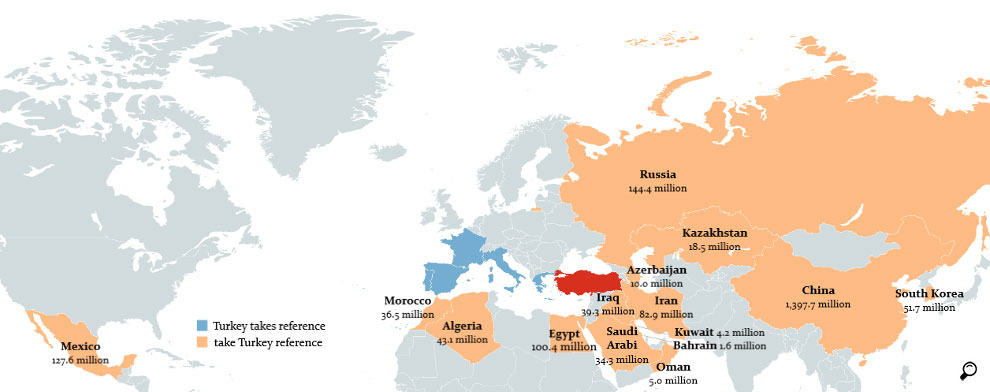
Similarly, the public price calculated after institution discount up to 41% on the published price in Turkey brings price levels of many new products well below the globally acceptable minimum prices, therefore reimbursement applications for new products face an impasse. As a result, in Turkey there are several registered but not marketed products as their reimbursement applications cannot proceed. It is important to draw attention to the fact that the underlying issue of this accessibility situation is the very low price levels imposed on medicines and that the issue requires urgent solutions.

