At a meeting held with the Minister of Labour and Social Security and the AIFD Board of Directors in 2017, the importance of enabling early reimbursement applications for products with ongoing licensing processes in order to accelerate access to innovative drugs was emphasised. With the approval of the Minister, with the support of the General Manager of General Health Insurance (GSS), the technical teams of GSS and AIFD came together to carry out common technical studies on the subject.
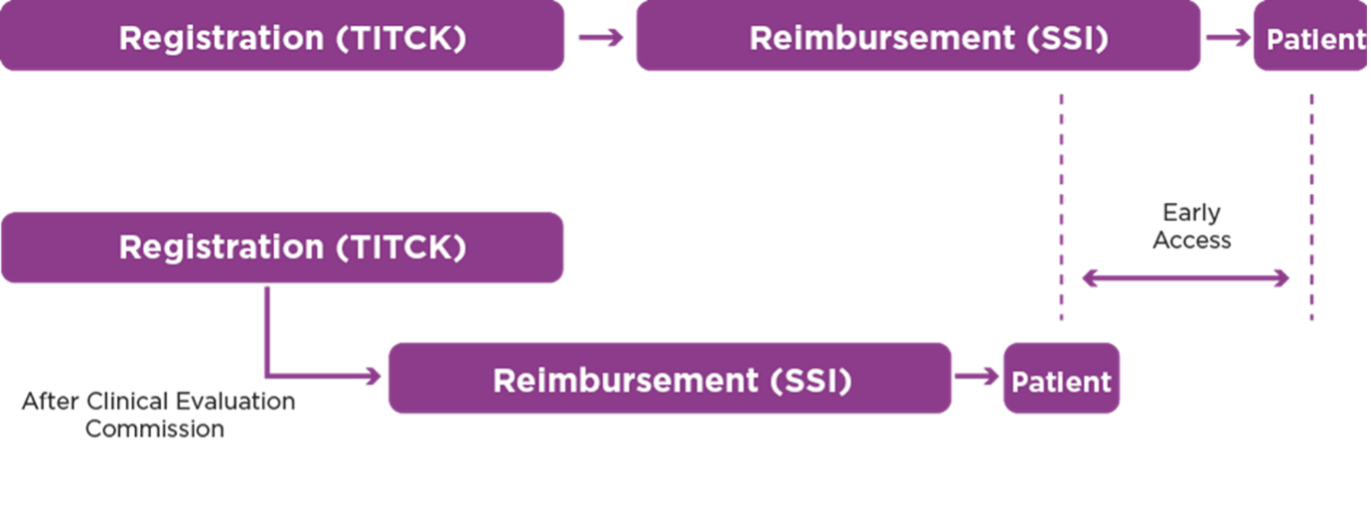
In order to shorten the time of the registration authorisation and reimbursement processes of innovative drugs, for the products that have "high priority" and "priority" in the registration application and that have already been approved by the clinical evaluation commission, it will be a crucially important improvement if simultaneous and parallel applications and evaluations could be made for early reimbursement application to the SSI (SGK). The access of the patients to treatments with “high priority” and “priority” products is delayed due to the time lag between the approval letter of the TITCK Clinical Evaluation Commission and the time for application for reimbursement to SSI.
It is considered that early application will not create significant risks in the IGOK (Medicines Reimbursement Commission) system, as a limited number of medicines would apply before the term, however, that can make, though limited nevertheless significant, contributions to the early access by the patients.