ACCESS TO INNOVATION
Compared with other countries, the access to innovative products in Turkey is below expectations. In a survey conducted by EFPIA (European Federation of Pharmaceutical Industries and Associations) the availability of the innovative molecules in different European countries after their approval between 2015-2018 by EMA (European Medicines Agency) is shown in the graphic below:
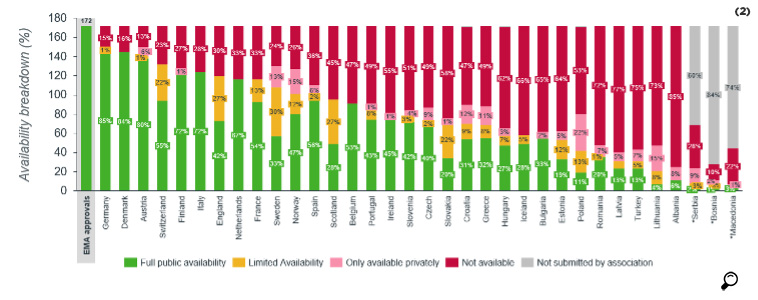
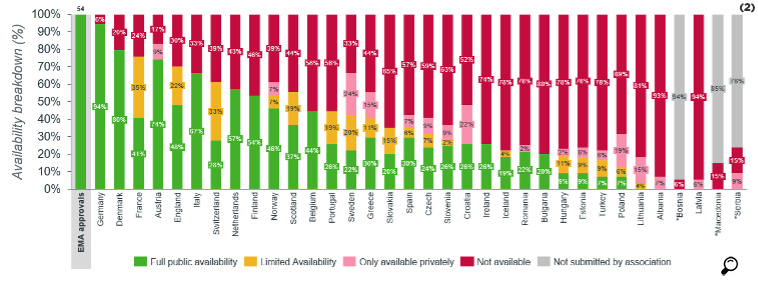
Out of the 172 medicines reviewed, 49% on average were available in the European Union countries, whereas the availability was 56% for oncology products. For Turkey, while for all products the availability was 18%, this ratio for oncology products was 26%. In 88% of the countries that were included in this survey, the availability of oncology products was superior compared to all products.
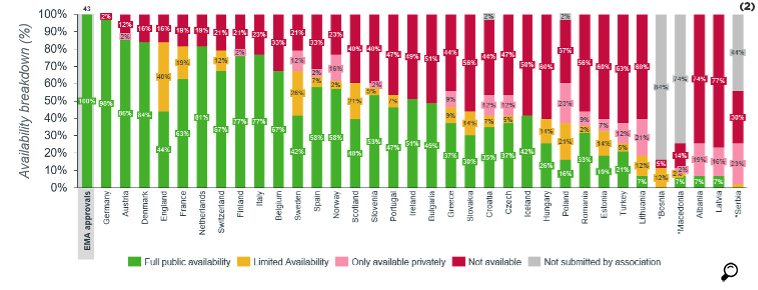
The availability of orphan drugs is 17% in Turkey whereas this ratio was 39% on average for the EU countries. In 80% of the countries, the availability of orphan drugs is much lower than the availability of all drugs. In 15% of the countries, the orphan drugs included in the survey were not reimbursed.
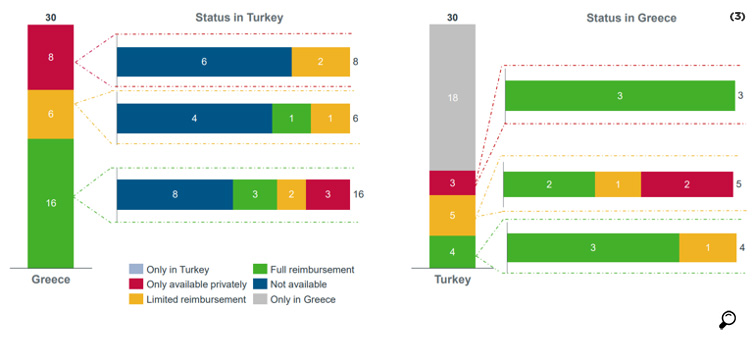
In a study conducted by IQVIA for AIFD, the availability of the 172 molecules used in EFPIA analysis was compared for Turkey and Greece. It was found that 25 of the molecules were reimbursed in both countries, while 44 molecules that were reimbursed in Greece were not reimbursed in Turkey.
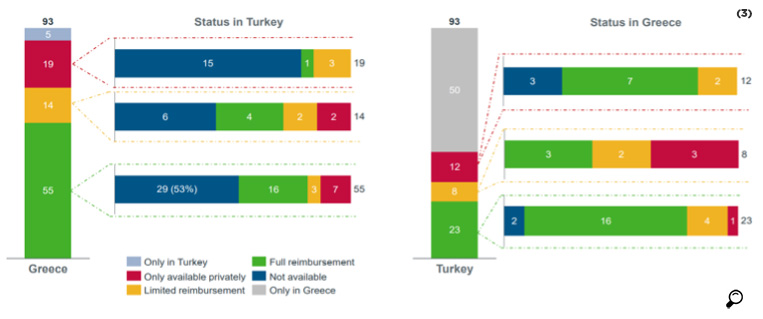
While out of 11 oncology products reimbursed in Turkey only one was not reimbursed in Greece, out of 20 products reimbursed in Greece 10 were not reimbursed in Turkey.
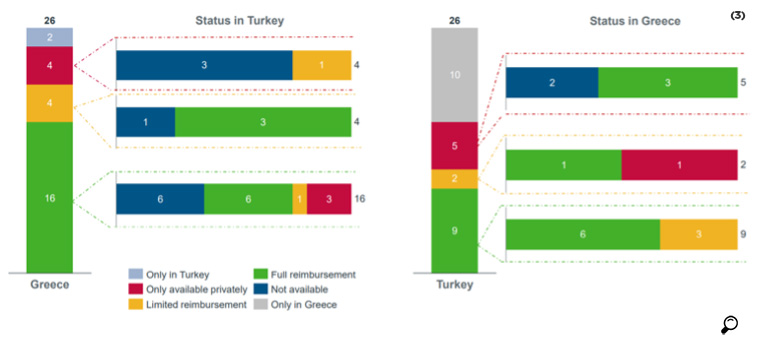
While out of 9 orphan drugs reimbursed in Turkey, 2 were not in the reimbursement list of Greece, out of 22 orphan drugs reimbursed in Greece, 15 were not reimbursed in Turkey.
According to another study conducted by IQVIA, out of new molecules marketed between 2017-2019, only 5% are available in Turkey. This ratio is 60% for the USA market and within the range of 30% to 40% for the European Union countries.
The most important reason for such low availability is the current pricing policy. To establish a sustainable pricing system the current spread of prices between Turkey and the reference countries should be improved gradually from the current 60% price multiplier. In order to encourage the registration of innovative products, a currency multiplier of 100% should be used in pricing innovative products.
Another important issue is the timely access to innovative products. According to the same IQVIA study, innovative medicines are introduced in Turkey with an average delay of 3 to 4 years. AIFD is developing policy recommendations in order to reduce the delays and to permit the patients in Turkey to access the innovative products in a timely manner as in developed countries.







