Pharmaceutical pricing in Turkey is conducted since 2004 with reference pricing system. According to the system, the prices of the pharmaceuticals are determined based on the lowest price of the five countries selected by the Ministry of Health (France, Spain, Italy, Portugal and Greece) and additionally the country from which the product is imported and the country where the “batch is released.”

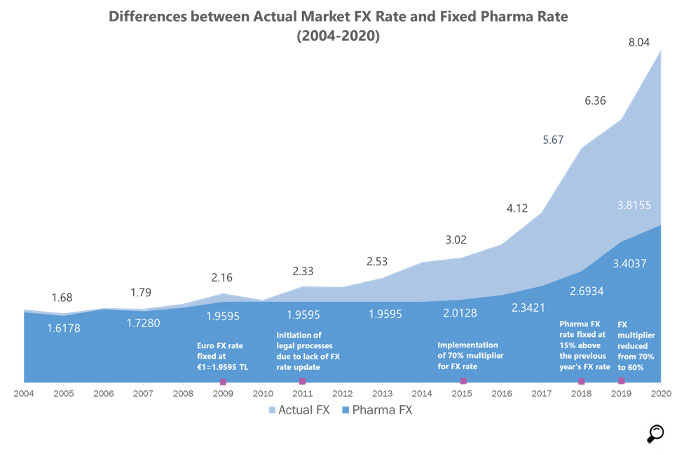
A fixed Euro rate announced by the Price Evaluation Commission is utilized each year to convert the Euro prices to Turkish Lira instead of the actual Euro rate in these countries. This fixed rate is determined based on related legislation of the Ministry of Health. According to the legislation on the pricing of pharmaceutical products, the annual fixed exchange rate is computed based on a certain ratio (multiplier) of the annual average Euro exchange rate of the previous year. 70% was the multiplier valid until 2018, that was updated as 60% in 2019. The value of 1 Euro for 2019 should have been 3.9710 Turkish Liras with the old multiplier, but the change in legislation introduced right before the validity of the new rate, put it at 3.4037 Turkish Liras. With this move, the real FX rate of Euro approached twice the value of the fixed pharmaceuticals exchange rate. For 2020, the fixed Euro exchange rate was announced as 3.8155 TL to 1 Euro.
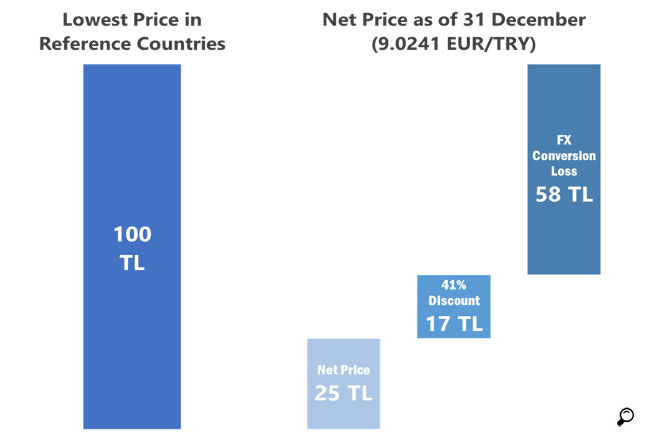
At the reimbursement stage, Social Security Institution additionally requires public institution discounts in addition to already low prices. Compared to Greece, which currently applies the lowest prices in Europe, the reimbursement prices in Turkey can be as low as a quarter of prices in Greece because of fixed rate and compulsory public institution discounts. These pricing policies are making the availability of innovative medicines to the patients and the sustainability of existing pharmaceuticals currently available in the market harder to achieve.